On Target Laboratories, Inc. has obtained US Food and Drug Administration (FDA) approval of an expanded indication for the use of Cytalux (pafolacianine) for the detection of lung cancer during surgery.
This biotech company discovers and develops targeted intraoperative molecular imaging agents to illuminate cancer during surgery.
In November 2021, Cytalux received FDA approval to assists surgeons in visually identifying additional malignant tissue in ovarian cancer to be removed during the operation.
Cytalux is the first and only targeted molecular imaging agent that can be used by surgical oncologists to illuminate lung and ovarian cancer intraoperatively.
“Today’s approval of an expanded indication for Cytalux marks an important step forward in the treatment landscape for lung cancer and for the more than 220,000 people in the US who receive a lung cancer diagnosis each year,” said Chris Barys, President and Chief Executive Officer of On Target Laboratories, Inc., in the company’s press release.
“We are proud to continue pioneering development of targeted intraoperative molecular imaging agents that illuminate cancers intraoperatively to enhance surgeons’ ability to see cancer in real time as they operate,” added Barys.
XTALKS WEBINAR: Patient Journey and Disease Progression — Exploring the Power of Images in Real-World Data
Live and On-Demand: Wednesday, January 25, 2023, at 1pm EST (10am PST)
Register for this free webinar to learn about use cases for imaging that include support for clinical trials, optimized dosing schedules and comparative effectiveness studies.
How does Cytalux Make Cancer Cells Glow?
Cytalux is intravenously infused into a patient one to 24 hours prior to surgery for lung cancer and one to nine hours prior to surgery for ovarian cancer. This imaging agent is comprised of a near-infrared dye and a ligand, or targeting molecule, that binds to folate receptors commonly found on many cancer cells. The accumulation of Cytalux on cancer cells makes these cells glow under near-infrared light, therefore surgeons can detect more cancer that might have otherwise been left behind.
Patients should not take folate, folic acid or other folate-containing supplements 48 hours before Cytalux administration because folic acid may decrease the detection of cancerous tissue with Cytalux.
The use of Cytalux carries a risk of misinterpretation as cancerous cells may not light up and non-cancerous cells might light up. Non-cancerous cells from other areas of the body may glow, such as areas of the kidneys, bowel, lymph nodes, lungs and inflamed tissue.
Clinical Trials of Cytalux (pafolacianine)
The initial FDA approval of Cytalux for assisting surgeons in identifying ovarian cancer lesions was based on a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer who were scheduled to undergo surgery. Out of 134 women who received Cytalux and were evaluated under both normal and fluorescent light during surgery, 26.9 percent had at least one cancerous lesion detected that was not found by conventional visual or tactile observation.
The recent FDA approval of Cytalux in lung cancer was based on the Phase III, multi-center, single dose, open label ELUCIDATE (Enabling LUng Cancer IDentification Using FolATE Receptor Targeting) trial. This clinical study included participants with known or suspected lung cancer who were scheduled to undergo surgery. Of the 110 participants who received Cytalux and were evaluated under both normal and fluorescent light during surgery, 24 percent had at least one cancerous lesion detected that was not found by conventional visual or tactile observation.
“During the ELUCIDATE trial, Cytalux proved to be a valuable surgical tool with its ability to localize lung lesions that may have otherwise been missed,” said Dr. Linda Martin, Chief of Thoracic Surgery, University of Virginia School of Medicine, in the company’s press release. “Cytalux has potential to become standard of care in thoracic surgery because of the safety and efficacy demonstrated in the trial. It is an important tool for surgeons to consider for patients.”
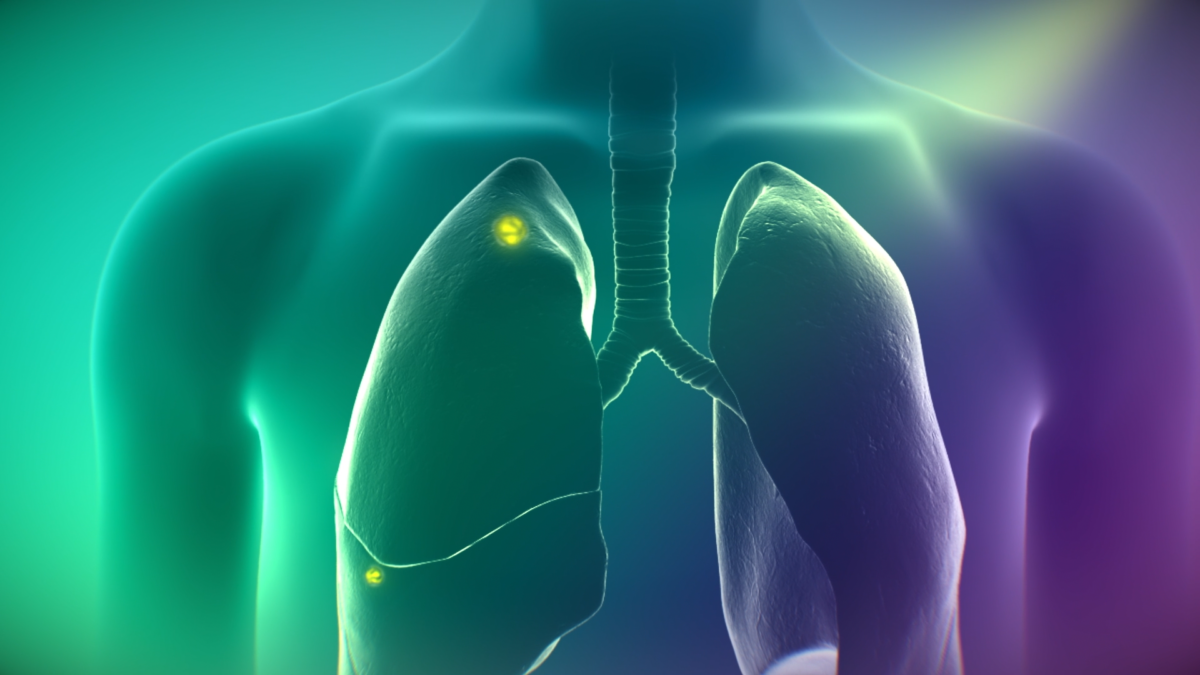
CYTALUX (pafolacianine) illuminating cancer in lungs. Photo courtesy of On Target Laboratories Inc.











Join or login to leave a comment
JOIN LOGIN