Johnson & Johnson (J&J) has submitted a New Drug Application (NDA) to the FDA seeking the first approval of icotrokinra for the treatment of adults and pediatric patients 12 years and older with moderate to severe plaque psoriasis.
Icotrokinra is designed to selectively block the interleukin-23 (IL-23) receptor, a key driver of inflammation in this disease.
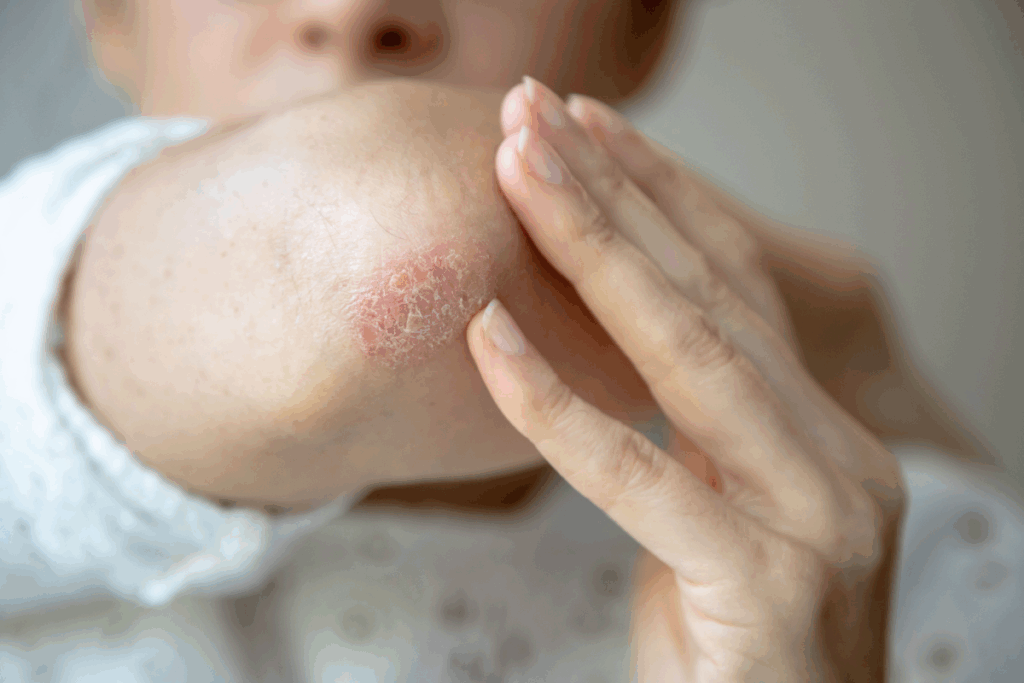
Plaque psoriasis is a chronic immune-related condition that causes skin cells to build up rapidly, forming inflamed, scaly patches called plaques. These raised areas can be itchy or painful and often appear on the scalp, knees, elbows and torso.
Approximately 8 million people in the US and over 125 million worldwide live with plaque psoriasis, with about one-quarter experiencing moderate to severe forms. This condition can affect quality of life, especially when plaques appear in sensitive or visible areas such as the scalp, hands, feet or genitals.
What Is Icotrokinra’s Mechanism of Action?
Icotrokinra is an investigational oral peptide that targets the IL-23 receptor, which plays a central role in triggering the immune response, causing inflammation in plaque psoriasis.
Unlike treatments that block the IL-23 molecule itself, icotrokinra binds directly to its receptor with very high affinity — measured in single-digit picomolar levels — meaning it strongly and selectively inhibits IL-23 signaling in immune cells.
The drug is taken orally once daily, providing a convenient alternative to injectable therapies.
XTALKS WEBINAR: Clinical Trial Application Success: CMC and Regulatory Insights from the US to Europe
Live and On-Demand: Wednesday, September 3, 2025, at 10am EDT (4pm CEST/EU-Central)
Register for this free webinar to gain practical insights on optimizing Investigational New Drug (IND) and Clinical Trial Application (CTA) submissions through aligned chemistry, manufacturing and control (CMC) and regulatory strategies.
How Did Icotrokinra Perform in Clinical Trials?
The NDA submission is based on data from four pivotal Phase III clinical trials in the ICONIC program: ICONIC-LEAD, ICONIC-TOTAL and ICONIC-ADVANCE 1 and 2.
ICONIC-LEAD evaluated overall efficacy and safety in adults and adolescents; ICONIC-TOTAL focused on psoriasis in difficult-to-treat areas; and ICONIC-ADVANCE 1 and 2 compared icotrokinra to both placebo and another oral therapy in moderate-to-severe plaque psoriasis.
Across these studies, icotrokinra met all primary and co-primary endpoints, demonstrating significant skin clearance and outperforming placebo.
ICONIC-LEAD enrolled 684 participants aged 12 and older, including adolescents, and evaluated skin improvement using two key measures: the Investigator’s Global Assessment (IGA) and the Psoriasis Area and Severity Index (PASI). The IGA is a five-point scale ranging from 0 (clear skin) to 4 (severe disease). The PASI score assesses the severity and coverage of psoriasis plaques, with PASI 90 indicating a 90% or greater improvement from baseline.
Icotrokinra showed higher rates of clear or almost clear skin (IGA 0/1) and PASI 90 at Week 16 compared to placebo.
ICONIC-TOTAL demonstrated strong skin clearance in difficult-to-treat sensitive locations. The ICONIC-ADVANCE 1 and 2 studies confirmed superior efficacy by achieving high rates of clear or almost clear skin in adults with moderate-to-severe plaque psoriasis.
Notably, the ICONIC-ADVANCE trials showed that icotrokinra was more effective than deucravacitinib, an approved oral treatment for moderate to severe plaque psoriasis.
Icotrokinra was generally well tolerated. Across studies, about 49% of patients taking the drug experienced adverse events compared to 52% in the placebo group, with no new safety concerns identified. Common side effects aligned with those typically seen in plaque psoriasis treatments, and no boxed warnings were reported.
In addition to these trials, J&J is conducting Phase III trials in active psoriatic arthritis (ICONIC-PsA 1 and 2) and a Phase IIb study in ulcerative colitis (ANTHEM-UC). The company has also launched ICONIC-ASCEND, the first-ever Phase III head-to-head study comparing an oral pill — icotrokinra — against an injectable biologic, ustekinumab. This study could offer new insights into the place of oral treatments compared with established injectables.
J&J plans to share detailed clinical data from the ICONIC program at upcoming medical meetings. The company has not yet announced specific plans regarding icotrokinra’s commercial launch or pricing.
If the FDA approval comes through, J&J’s new investigational therapy could offer a convenient oral option with a novel mechanism of action and promising clinical benefits.
Related: Starjemza (Ustekinumab) FDA Approval: 2025’s First Biosimilar for Stelara in Psoriasis Care
Psoriasis Treatment Innovations: Who Else Is on It?
In recent months, several companies have been advancing new therapies for psoriasis in clinical development.
Ascletis has received FDA clearance to begin Phase I testing of ASC50, a novel oral inhibitor targeting the IL-17 pathway, aimed at reducing inflammation in plaque psoriasis.
Accropeutics is developing AC-17, a small molecule designed to modulate immune responses involved in psoriasis, currently progressing through early-stage trials.
Akeso Bio recently announced positive preclinical results for AKS-830, an antibody targeting a novel immune checkpoint, with plans to initiate clinical trials in patients with psoriasis.
Oruka Therapeutics secured FDA clearance to start a Phase IIa trial of ORKA-001, a topical treatment for plaque psoriasis, with promising Phase I data scheduled for presentation later this year.
In industry news, Boehringer Ingelheim and LEO Pharma will partner to commercialize and further develop spesolimab, an antibody targeting inflammatory pathways for skin diseases. This collaboration hopes to accelerate the availability and advancement of spesolimab for patients with chronic plaque psoriasis and related conditions.
All in all, these developments target diverse pathways for psoriasis treatment.
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN