UK-based med tech company LivaNova announced positive results from its OSPREY clinical study evaluating the LivaNova aura6000 system for the treatment of obstructive sleep apnea (OSA) using targeted nerve stimulation.
The study successfully met its primary safety and efficacy endpoints.
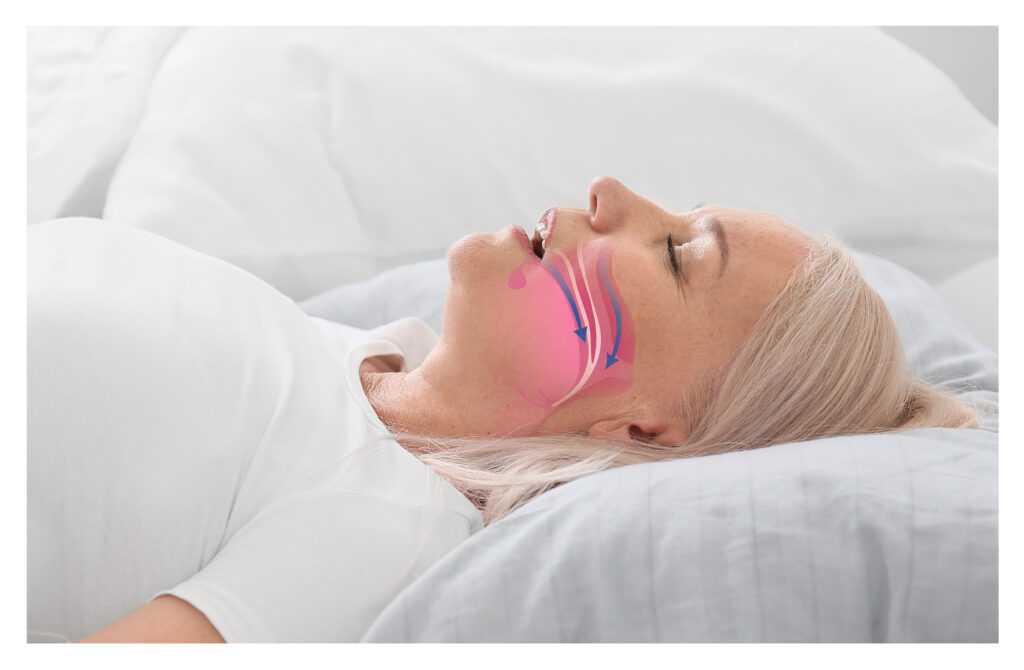
The aura6000 is an implantable hypoglossal neurostimulator designed to treat adult patients with moderate to severe OSA.
The hypoglossal nerve, also known as cranial nerve XII, is the twelfth and final cranial nerve. It plays a crucial role in controlling tongue movements, which are essential for speech, swallowing and maintaining an open airway.
OSA is a sleep disorder where the airway repeatedly becomes partially or completely blocked during sleep, causing breathing to stop temporarily. This results in fragmented sleep, loud snoring and often excessive daytime sleepiness, which increase the risk of health issues like high blood pressure, heart disease and stroke.
XTALKS WEBINAR: Unlock Growth in Medical Device Manufacturing with an AI-Powered eQMS
Live and On-Demand: Monday, December 9, 2024, at 11am EST (4pm GMT/UK)
Register for this free webinar to unlock the potential of AI in quality management and propel medical device manufacturing into the future.
The OSPREY clinical study was designed to assess the safety and effectiveness of LivaNova in individuals with moderate to severe OSA who have failed or are unwilling to use positive airway pressure treatment.
In the randomized controlled study, statistical significance was achieved for the primary endpoint of responder rates between the treatment and control arms.
LivaNova is focused on developing products and therapies for the “head and heart,” according to the company. Its product portfolio and pipeline include treatments for cardiopulmonary conditions, difficult-to-treat depression, drug-resistant epilepsy and OSA.
In the trial, reductions in the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) were analyzed as part of the study’s secondary endpoints.
The primary endpoint includes achieving a response rate that reflects a 50-percent improvement from baseline AHI. A resulting AHI value below 20 was also a critical measure in the study.
In the analysis, at the seven-month mark, median values from baseline to six months with therapy were assessed.
Participants in the group who received neurostimulation with the aura6000 device experienced a 66.2 percent reduction in AHI when the median at baseline (34.3) was compared to the median at six months (11.6).
ODI was cut by 63.3 percent, with a median of 12.8 at six months compared to 34.9 at baseline.
LivaNova said no serious adverse device-related or procedure-related events were reported throughout the primary endpoint visits.
Related: How GE HealthCare’s SIGNA MAGNUS 3.0T Transforms Neuroimaging
In a news release announcing the results, Vladimir Makatsaria, CEO of LivaNova, stated: “The study results reinforce our belief that targeted hypoglossal nerve stimulation provides a compelling alternative for patients with obstructive sleep apnea. The significant reductions in AHI and ODI achieved after only six months of therapy gives us strong evidence of this technology’s potential at 12 months and beyond.”
He said the company is looking forward to the full results at the 12-month mark for patients receiving the therapy. This long-term data will be evaluated at the 13-month follow-up visit, which LivaNova expects to be available in the first half of 2025.
The company said once the latest six-month results analysis is complete, the company will submit the OSPREY clinical data to the US Food and Drug Administration (FDA) as part of its premarket approval submission for aura6000.
The FDA granted 510(k) clearance last year for LivaNova’s Essenz heart-lung machine, for use in cardiopulmonary bypass procedures.
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN