Medtronic has announced the FDA clearance for its Hugo robotic-assisted surgery (RAS) system for use in urologic surgical procedures, as the company seeks to expand its footprint in the fast-growing surgical robotics market.
The clearance opens the door for the Hugo system to be used in a range of urologic procedures, an area that represents one of the largest segments of robot-assisted surgery globally.
It also positions Medtronic as a more direct competitor to Intuitive Surgical’s long-dominant da Vinci platform.
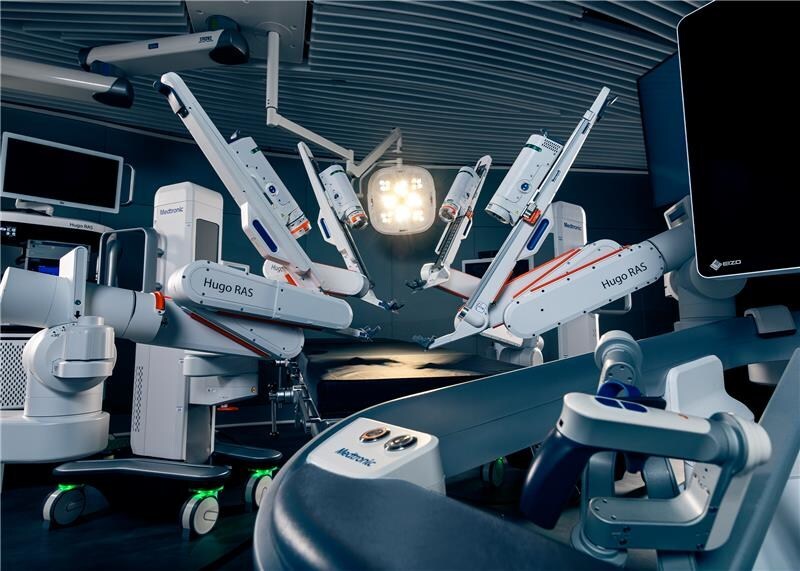
Medtronic launched the Hugo system with the goal of increasing accessibility to robotic surgery through modular design, improved ergonomics and potentially lower operational costs for hospitals.
Related: Pixee’s Knee NexSight for Augmented Reality-Based Surgical Navigation Cleared by FDA
“This is an incredibly exciting day for healthcare in the United States. FDA clearance of the Hugo RAS system means there is now choice for hospitals looking to expand their robotic programs and increases access for patients,” said Rajit Kamal, vice president and general manager of Robotic Surgical Technologies within the Surgical business of Medtronic.
“As we begin our purposeful launch of the Hugo RAS system in the US, our focus is on building a strong foundation with leading hospitals through our differentiated approach to partnership, rooted in our enduring commitment to provide an excellent customer experience and enable surgical teams to deliver the best possible outcomes for their patients.”
The Hugo system is designed to offer several clinician- and hospital-focused advantages, among which is a modular architecture that enables flexible operating room (OR) configurations.
The independent robotic arms are designed to enhance precision and reduce arm collisions, while 3D visualization and advanced instrumentation support complex, minimally invasive procedures.
Common minimally invasive procedures include prostatectomy, nephrectomy and cystectomy, which account for about 230,000 surgeries every year in the US, according to Medtronic.
There are also potential cost efficiencies aimed at broadening access for health systems with budget constraints.
Medtronic has positioned Hugo as a more flexible and potentially more cost-effective option, especially for mid-sized hospitals and community centers that have previously been priced out of robotics programs.
XTALKS WEBINAR: Medical Device Validation Solutions for Evolving Industry Needs
Live and On-Demand: Wednesday, January 21, 2026, at 1pm EST (10am PST)
Register for this free webinar to learn why Computer Software Assurance (CSA) demands a rethink of traditional validation for medical devices.
“The Hugo RAS system represents a new and exciting approach to robotic-assisted surgery,” said Dr. James Porter, a urologic surgeon and chief medical officer for Robotic Surgical Technologies and Digital Technologies within the Surgical business at Medtronic. “We’re excited for surgical teams in the US to experience the differentiated technology and partnership from Medtronic, which supports them at every stage of their robotic surgical journey.”
The Hugo RAS system successfully met its primary safety and effectiveness endpoints in the Expand URO investigational device exemption study, the largest multi-port robotic-assisted urological surgery trial conducted in the US. The results were in line with outcomes reported in the broader clinical literature.
The clearance represents the first major regulatory green light for Hugo in the US following its international rollout across Latin America, Asia-Pacific and Europe over the last several years.
According to Medtronic, globally, the Hugo platform has already been used in tens of thousands of urologic, gynecologic and general surgery procedures across more than 30 countries on five continents.
Medtronic is expected to accelerate its US commercial rollout and plans to build on its US entry by pursuing additional indications, with clearances for general and gynecologic surgery expected to follow the initial urology approval.












Join or login to leave a comment
JOIN LOGIN