Emerging obesity therapies are rapidly redefining how weight-management studies are designed, measured and interpreted. As scientific understanding of obesity deepens, researchers are now looking beyond the number on the scale and are focusing instead on durability (how long results last), body composition (the balance between fat and muscle) and overall quality of life.
In a recent Medpace webinar, experts Dr. Phillippa Miranda, Dr. Douglas Lee, Dr. Salvatore Zabbatino and Andy Hood discussed how next-generation obesity trials are adapting to this fast-changing therapeutic landscape. The conversation spanned everything from GLP-1-based combination therapies to lean-mass preservation, imaging methodologies and functional digital endpoints.

The speakers noted that obesity research has moved beyond a narrow focus on weight reduction toward a broader emphasis on metabolic health, durability and patient quality of life. They also pointed out that age-related muscle loss is a growing global concern, making it an increasingly important consideration in next-generation obesity trial design.
Redefining Success in Obesity Therapy
Dr. Miranda said it’s important to reframe what success in weight management truly means today.
“The first goal of weight management is really to achieve an adequate amount of weight loss,” she said, noting that targets have traditionally been around 5% to 10% of total body weight (Figure 1). “But with the success of many GLP-1-based therapies, we now see people aiming for more than 15% or even 20% weight loss.”
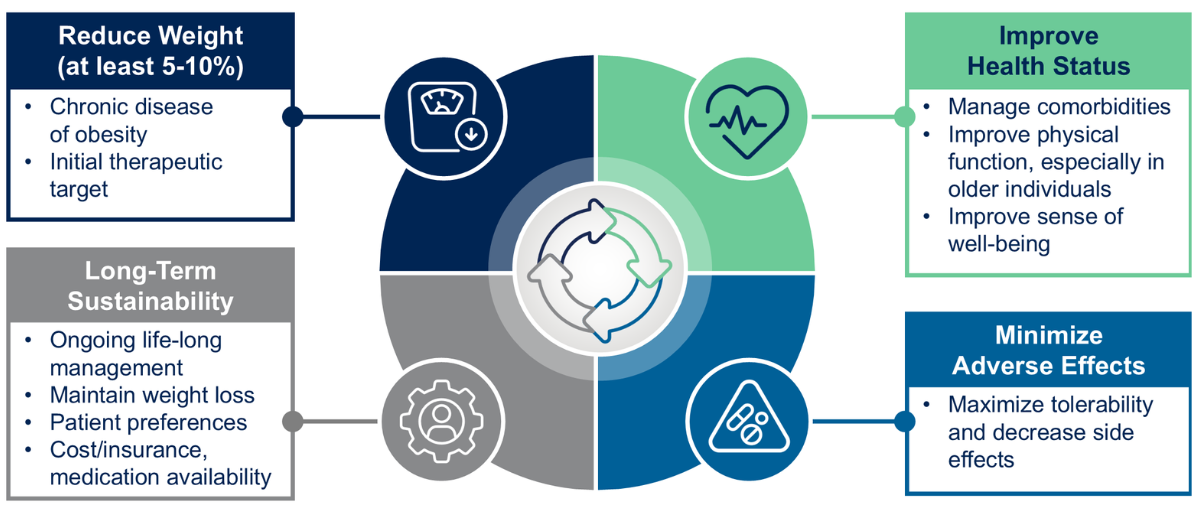
Beyond numbers, she emphasized that modern obesity therapies must demonstrate improved health status, minimized side effects and long-term sustainability. “Patients want to feel better and maintain their weight loss,” she added. “It’s really about the quality and durability of the outcome.”
The Rise of GLP-1 Therapies and a Shifting Trial Landscape
Hood outlined how GLP-1 receptor agonists have transformed the obesity landscape.
Prescription data from 2019 to 2024 showed a fourfold rise in GLP-1 use, with US population coverage projected to go over 9% by 2030. Medicaid prescriptions alone grew by more than 400% between 2019 and 2023.
There has also been a sharp rise in obesity-related clinical research, from 164 trials in 2020 to over 500 by 2024 (Figure 2), with GLP-1 mechanisms representing roughly half of new studies. Combination-therapy designs expanded fivefold, signaling a clear pivot toward multi-agent strategies.
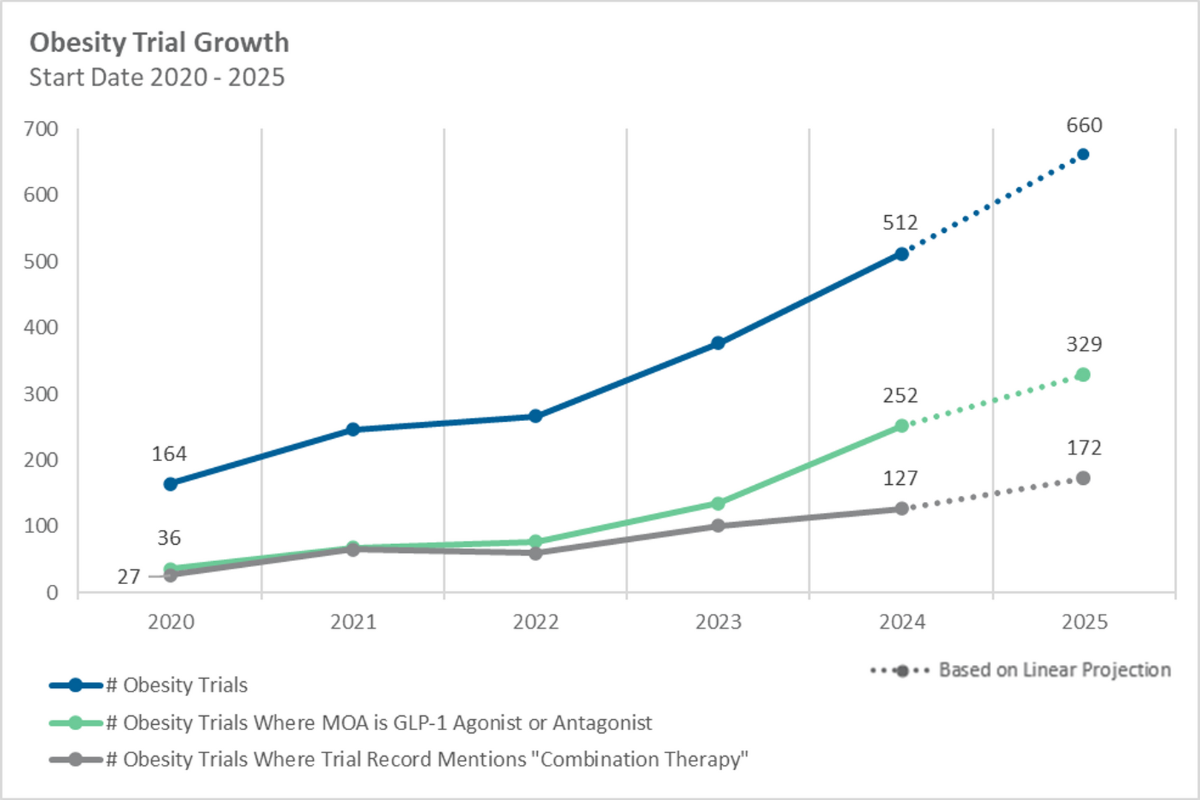
Hood explained that while early trials focused heavily on weight reduction, the surge of GLP-1 therapies has shifted the emphasis toward combination regimens and maintenance strategies.
“Maybe you’ve hit your initial weight loss goal,” he said. “What are you doing at that point? Are you switching to a different maintenance drug, or combining your therapy with a GLP-1-based agent?”
Dr. Miranda noted that the surge in GLP-1-based and combination studies is prompting sponsors to rethink trial design. Beyond traditional placebo-controlled weight-loss models, new approaches such as switch designs are helping assess maintenance and tolerability in patients already using GLP-1s. These “switch” studies let researchers see what happens when patients transition from one treatment to another, an approach that mimics real-world prescribing patterns.
Meanwhile, combination-therapy studies are gaining traction as a way to enhance efficacy and reduce side effects.
Within this broader trend, Dr. Miranda pointed out that a major focus of next-generation trials is lean-mass preservation, shifting the emphasis from how much weight is lost to how that weight is lost.
From Weight Loss to Muscle Preservation
Dr. Lee explored why muscle preservation has become a pivotal goal in obesity drug development.
He began by explaining how age-related muscle loss, or sarcopenia, can start as early as a person’s 40s and progress by 1% to 2% annually after age 50. Sarcopenia affects roughly 10% of Americans over 55 and twice that proportion in Japan. Over time, this can lead to sarcopenic obesity, a dual challenge of excess fat and declining muscle mass.
“There have been concerns about the potential impact of GLP-1 receptor agonists on sarcopenia,” said Dr. Lee. “That’s why lean-mass preservation studies are becoming more common, especially in high-risk and older populations.”
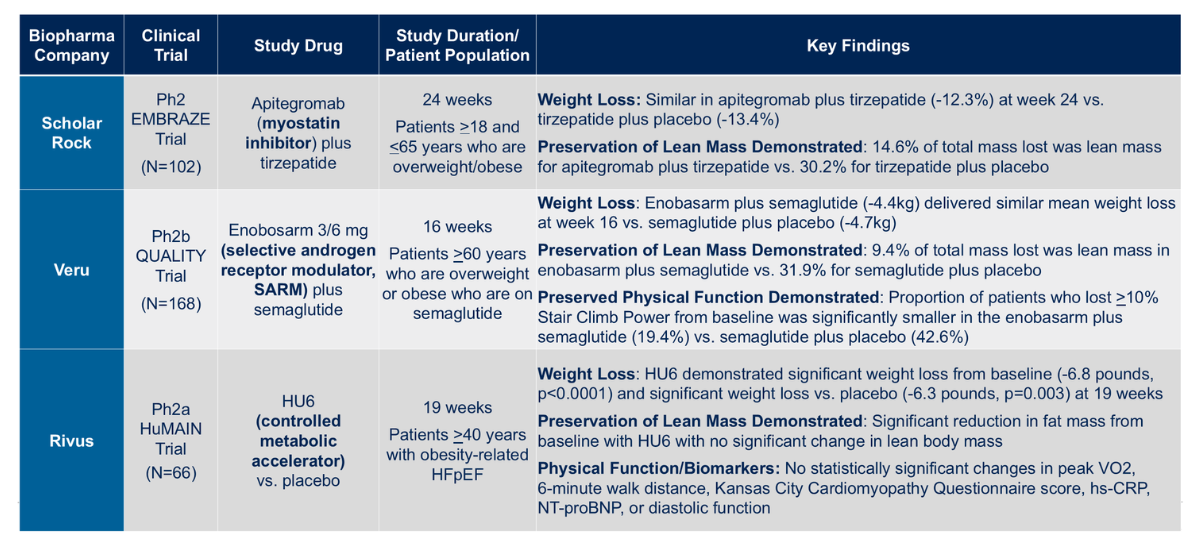
He then reviewed three key trials (Figure 3):
- Scholar Rock’s EMBRAZ study: a 24-week trial adding the myostatin inhibitor apitegromab to tirzepatide, which successfully preserved 15% more lean mass compared to tirzepatide alone
- Veru’s QUALITY study: using enobosarm, a selective androgen receptor modulator, combined with semaglutide, preserved about 20% more lean mass and significantly improved physical function
- Rivus’ HuMAIN study: tested HU6 in patients with obesity-related heart failure with preserved ejection fraction (HFpEF), showing reduced fat mass without significant loss of lean mass
In addition, Eli Lilly’s BELIEVE Phase II trial combined semaglutide with bimagrumab, a dual ActRII antibody blocking activin and myostatin pathways. The regimen achieved 22.1% weight reduction, driven by more than 90% fat-mass loss and a 2.5% increase in lean mass (Figure 4). Improvements in SF-36 [Short Form-36 Health Survey, a questionnaire measuring physical, mental and general wellbeing] physical and mental domains suggested both metabolic and functional benefits.
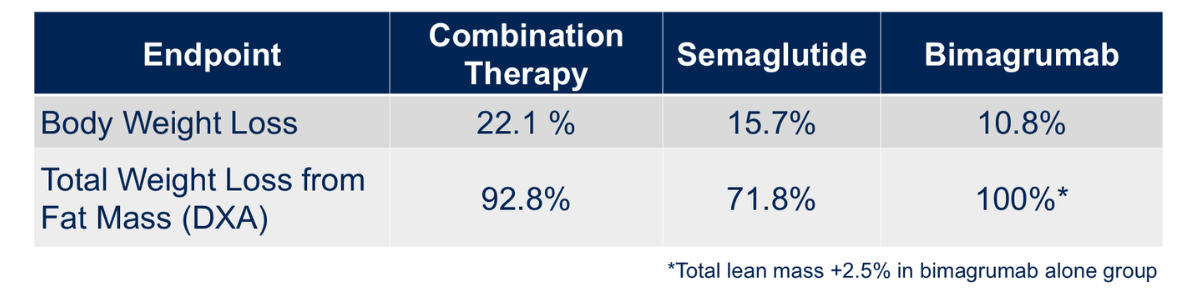
Dr. Lee called the BELIEVE trial “very successful,” pointing out that “in the bimagrumab arm, there was actually a 2.5% increase in total lean mass.”
Imaging Tools for Body-Composition Studies
Dr. Zabbatino provided insight into imaging technologies that underpin these lean-mass preservation trials.
He explained that DXA [dual-energy X-ray absorptiometry] remains the preferred method due to its low radiation exposure, wide availability and cost efficiency. It offers the ability to segment the body into fat, lean and bone mass and can even calculate visceral (VAT) and subcutaneous adipose tissue (SAT) levels.
“With DXA, even if a patient is too large for the standard field of view, offset imaging can be used to ensure reliable measurements,” he said.
By contrast , MRI can map fat and muscle volumes more precisely (Figure 5). Though it might be less practical for full-body scans due to cost, time and accessibility. “Patients with joint replacements or pacemakers may be contraindicated,” Dr. Zabbatino explained. “And operationally, MRI access can vary widely by region.”
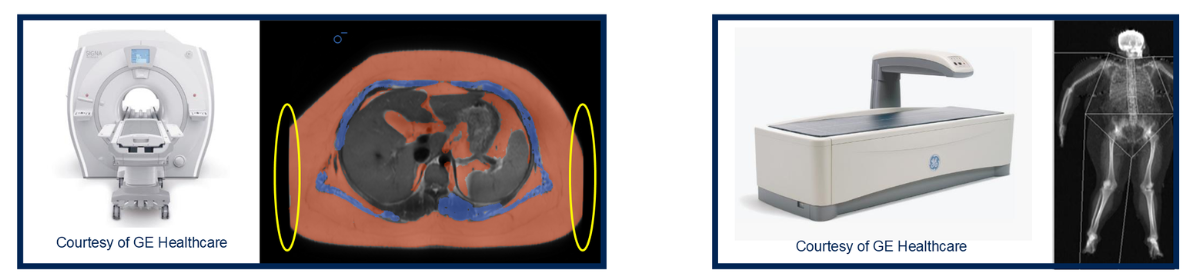
He added that MRI can still play a valuable role in sub-studies, especially for quantifying muscle quality or hepatic fat fraction, helping sponsors correlate structural changes with clinical outcomes.
Switch-Design Studies: Beyond Initial Weight Loss
Dr. Miranda discussed switch-design trials, which explore what happens when participants transition from one therapy to another, often from a GLP-1 to a new investigational product.
“The basic concept is to randomize participants who are stable on a prior therapy,” she explained. “You can then assess whether switching improves maintenance, tolerability or metabolic outcomes.”
Such studies are increasingly common as obesity therapies mature and the patient population diversifies.
Hood noted that operationally, this design comes with unique retention challenges. “Five years ago, finding enough patients who were already on stable GLP-1 therapy was difficult,” he said. “Now that these drugs are more widely used, switch studies are not only feasible but essential.”
He added that patient psychology also plays a major role: “If there’s any weight rebound or the regimen feels harder to maintain, participants can lose motivation. Having support systems and trial processes in place to sustain engagement is critical.”
These trials, he explained, help evaluate not only ongoing efficacy but also patient preference, convenience and quality-of-life metrics.
Beyond Weight: Functional, Metabolic and Cardiovascular Endpoints
Dr. Zabbatino highlighted how new endpoints now capture the real-world benefits of weight loss and maintenance therapies (Figure 6).
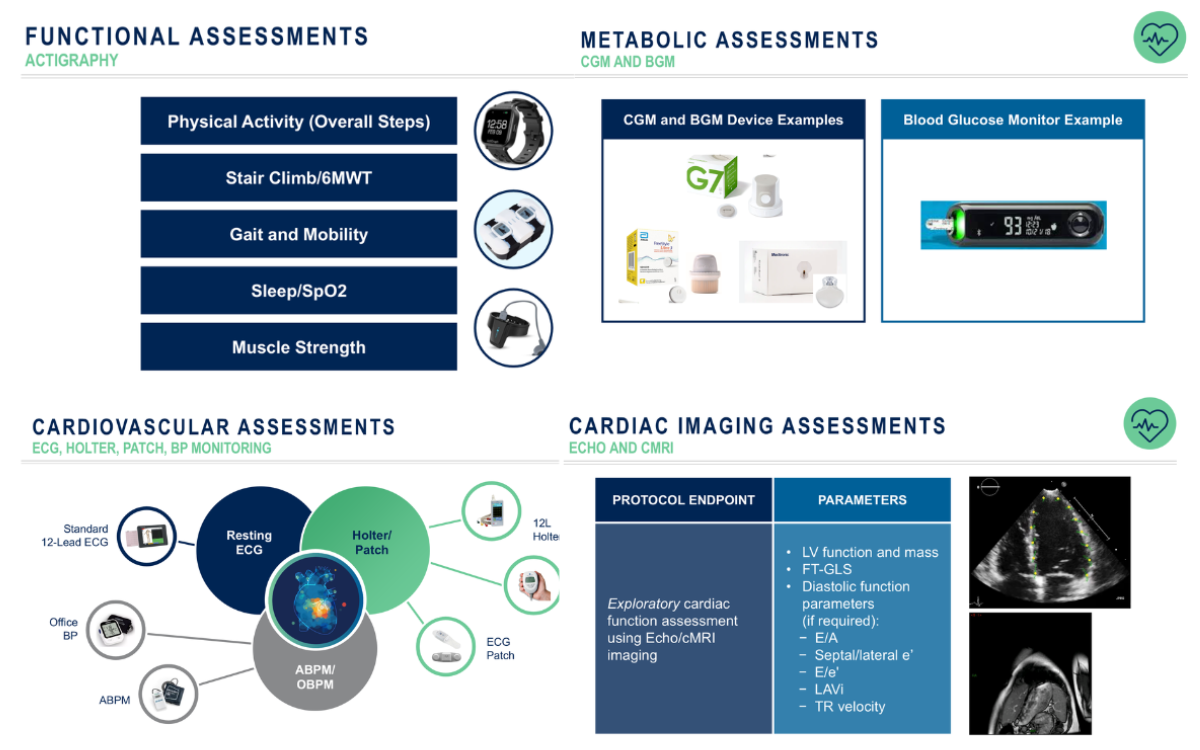
“We’re losing weight, but what does that mean in terms of what’s going on?” he asked. “Functional assessments like actigraphy and six-minute walk tests give us insight into daily mobility, muscle strength and endurance.”
Other endpoints include sleep quality, oxygen saturation and joint function, all directly impacted by weight and metabolic improvements.
“Continuous glucose monitoring [CGM] and ambulatory blood pressure monitoring [ABPM] can provide seamless metabolic and cardiovascular data without disrupting a patient’s lifestyle,” he added.
Dr. Zabbatino also emphasized integrating imaging and biomarker data, such as echocardiograms (ECHO) and cardiac MRI, to assess heart health improvements.
He said that the integration of imaging, biomarkers and digital health tools will continue to refine how therapies are evaluated in real-world conditions. “There’s a lot to be done,” Dr. Zabbatino said, “not just in weight, but in understanding how we’re improving quality of life.”
Hood reflected on how the obesity clinical research field has shifted.
“We’ve seen a move away from focusing purely on weight reduction,” he said. “Now it’s about maintaining outcomes, preserving lean mass, improving overall health and making sure patients can sustain their progress.”
In the Medpace webinar, the expert panel painted a clear picture of where obesity clinical development is headed: toward precision, sustainability and holistic health outcomes. As therapies become more effective and diverse, next-gen trials must balance scientific complexity with practical design, thus ensuring that advances in imaging, data collection and patient engagement lead to lasting, real-world benefits.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN