Executing a successful clinical trial involves the effective integration of a number of different factors, which Medpace and the Batten Disease Support and Research Association (BDSRA) partnered together to discuss in this webinar. Some of these considerations include appropriate study design, protocol development, partnerships with patient advocacy groups and of course, patient selection, retention and recruitment.
One of the keys to a robust clinical study is optimal patient participation. Given this, it has become increasingly important to design trials that are patient-centered. Moreover, it is not only important to engage patients themselves, but also their family members and caregivers because they are an essential part of patients’ lives as they provide care and serve as advocates for the patient.
Designing clinical trials for rare diseases can be particularly challenging as the trial groups are typically relatively small populations with specific and unique needs. For rare disease trials, it is therefore necessary to work closely with patients and their caregivers in order to ensure that they are effectively educated, supported and engaged throughout the trial. This helps foster greater adherence and successful trial completion.
Miaesha Campbell from Medpace and Tauna Batiste and Noreen Murphy from BDSRA discuss the significance of identifying the unique needs and goals of patients and caregivers in clinical trials for rare diseases in their webinar. They discussed the importance of engaging pertinent advocacy and patient-caregiver groups through outreach strategies, appropriate communication tools and education. Patient-centered approaches are necessary to ensure that patients and their caregivers are supported and engaged at each stage of a trial.
Optimizing Patient Engagement
It is important to engage appropriate groups early on in the trial process, which begins right at the stage of designing the trial. These groups include patient advocacy groups, clinical teams that include the principal investigator (PI) and study coordinators, in addition to patients themselves and their caregivers.
Noreen Murphy, Patient and Family Education Coordinator at the BDSRA says that by becoming “proactive partners within these communities, we can make sure that our strategies are implemented across our organization but also that we consistently keep in mind how our clinical research studies will affect members of the community and/or their caregivers.”
Study coordinators should facilitate active communication and serve as a liaison between these groups to ensure the smooth execution and operation of a trial. This must begin at the patient, keeping their needs, goals and outcomes in mind and at the center of any trial design. This is achieved by engaging key players early on in the process so that particular needs and elements can be incorporated into the study design itself.
By facilitating the active participation of patients and their caregivers in rare disease trials, patients will be more motivated to participate and be active players in the trials, leading to greater compliance, retention and overall trial success.
Advocacy Engagement
To provide patients with the needed education, guidance and support, advocacy is key to ensuring that the unique needs of patients with rare diseases are met when setting up and conducting trials. Both patients and their caregivers need to be fully engaged and involved in the process.
One of the key goals of advocacy engagement is to become a member of the community. By going into the community, study personnel (i.e. coordinators) can interact and communicate with advocacy, patient and scientific/medical groups to gain information and insight into the goals and needs of the groups, which will inform appropriate trial protocol development.
It is important to communicate with patient advocacy groups as they have a deep understanding of the specific rare disease communities that they work with. These advocacy groups should be engaged early to inform them about the clinical trial and how it is relevant to their patient populations. By forming partnerships, the trial can be advertised through their communication channels, which can include listing the trial on their website, sending e-blasts/newsletters to patients and through webinars.
In addition to advocacy groups, patient communities should also be directly engaged. In today’s digital age, patients are highly interconnected through online groups, forums and social media. These are therefore great places to engage patients to let them know about upcoming trials and provide them with relevant information.
Moreover, patients often attend conferences to keep up-to-date on the field of their rare disease, meet with expert scientists and clinicians as well as fellow patients. Meeting patients and their caregivers at conferences and events is an ideal means of engagement.
Identification of Patient and Caregiver Needs, Support and Goals
“By becoming a member of patient communities, the needs of both patients and caregivers can be better understood,” says Miaesha Campbell, Director of Patient Recruitment at Medpace. Having a rare disease comes with a great deal of burden not only on the patient suffering from the disease, but also on caregivers who provide physical, emotional and mental care for the patient. Therefore, CROs and study trial coordinators must be mindful of the impact of their trial on both the patient and caregiver(s). For example, “during a trial, there may be extra burden placed on caregivers as they may need to take time off of work in order to take the patient to visits and appointments associated with the trial,” says Campbell.
According to Campbell, it is important to “bring some focus and patient-centricity from a digital aspect, so we encourage our partners to go beyond just listening to the patient needs that come from an assessment, but really focus in on what their goals are.”
It is thus critical to identify the goals of patients and caregivers in a trial. These goals can vary from patient to patient, and from patient to caregiver. For example, a patient may volunteer to participate in a trial in hopes of an experimental treatment helping them feel physically better (i.e. restoring eyesight), while others may be interested in how it helps change their behavior (i.e. dealing with daily activities).
Some patients “may be born with the disease while others may have developed the disease later on in life and are coping with the associated changes at each stage of the disease, as well as their life,” says Campbell. The goals of patients can thus be very different, which is why it’s important to identify them. It is necessary for patients and caregivers to understand what a trial may do for them because it is with this understanding that the patient will ultimately decide if the study is beneficial to them, which ultimately impacts enrollment, adherence and trial success.
Technologies can be used to help reduce trial burden and make sure that patients have a positive trial experience as they are volunteering their time and effort. It is important to consistently communicate with patients and caregivers both before and throughout the course of a trial to ensure that they are as knowledgeable and comfortable as possible. This can involve providing midway reports, communication pieces and importantly, asking for feedback.
Connecting with patient groups in person, within communities or online is important in developing strong relationships based on trust and knowledge.
“There are different types of online communities and so how do we connect with those groups, who are those groups and making sure that we understand exactly what those needs are and that again, it’s about an ongoing relationship,” says Noreen Murphy, with respect to engaging patients and their caregivers through fostering an online presence.
She continued to say that by “attending the actual advocacy group conferences for patients, you get real-world feedback from these patients in terms of how you can best support them.”
Patient-Centricity
Placing patients at the center of a trial can help yield successful outcomes in terms of study design, patient participation, adherence, satisfaction and study results. Having a patient-centered approach can increase awareness, reach and enrollment.
This can help in connecting with caregivers and key support/advocacy groups, in addition to supporting the patient journey through education and building relationships. This can be achieved by listening to patient needs, understanding the journey of the patient and caregiver, incorporating patient needs in trial development and execution, along with assessing patient burden and impact on parents/guardians/caregivers.
It is important to engage patients and their caregivers in order to find effective ways to offer support and give them information in a clear manner. Having a clear message comes with good planning, which is why it is important to have a clear plan and build solid relationships with patients and advocacy groups early on.
Fostering strong relationships with patients, caregivers and patient advocacy groups needs to begin early on in the trial design and process. Developing relationships is important not only for understanding what is needed for the trial, but also what patients need.
Engaging patients and caregivers can be achieved by increasing reach into communities, attending conferences and engaging focus groups where patients can look at study protocol designs and/or materials. This is to make sure that your message is being clearly communicated and importantly, is resonating with them so that they actually feel motivated to participate in the study.
Patient and caregiver engagement is critical in helping inform study design, and in adapting protocol designs based on real-world patient feedback. Therefore, planning ahead is important to ensure that patients have a positive and less-burdensome clinical trial experience.
Study designs, expectations and tools should be prioritized in order to have sufficient time to build patient awareness and for development tools to be ready upfront. These can be in the form of digital tools and a strong online presence as patients are actively using the internet in their personal research. Clear and appropriate reimbursement procedures are also important in motivating patients to sign up for a trial.
Multifactorial Approach to Awareness
Having a diversified and multifactorial approach to building awareness and providing education and support to patients and their caregivers translates into a more informed patient. This involves a combined approach that incorporates advocacy and registry groups, educational materials and digital awareness.
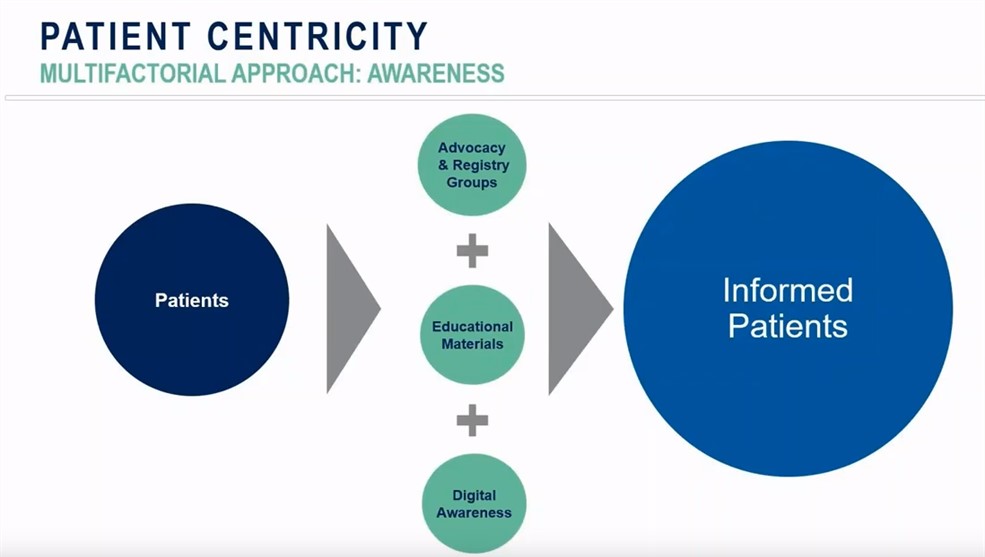
Tauna Batiste, Executive Director of the BDSRA, stressed the importance of a multifactorial approach, saying that “we consider a multifactorial approach to awareness where you have to bring in all stakeholders, but you also have to make sure that you have your supportive items ready; you have to really start thinking about it and planning for it upfront. You have to think about the patients, you have to develop those relationships with advocacy groups, the patient group and even registries, understanding exactly where the patients are.”
Advocacy Groups: Partnering with advocacy groups early helps in the planning and provision of education and supportive items. It is important to have advocacy groups involved as they have a specific focus on patients, their families and caregivers. Because these groups have already been working closely with these stakeholders, it is easier to convey information and communicate with patients and families through the advocacy groups, who can interact with them in a way that puts them at ease.
Educational Materials: To help with recruitment and engagement, having information on trials readily available online and through advocacy groups is essential to the process. Site-specific recruitment goals involve the identification of patient pathways in terms of their external networks through which they can learn about trials (i.e. via advocacy groups or patient conferences). These are ways in which a patient is going to be made aware of a study so it is important to engage and support these efforts early and make information available to them that is clear and easy to understand.
Digital Awareness: Using technologies and integrating different assessment tools such as online resources (websites, social media), patient handbooks, apps and tablets/iPads is an appealing way to share information. The idea is to provide information that is not only clear, but also intuitive and accessible because it is more likely to be utilized. Moreover, educational tools can be used for both awareness and retention. For example, patients can record their patient journeys using photo albums. In addition, apps can be used to monitor appointments, keep logs and have information on the trial readily available to patients and caregivers. These are convenient and less burdensome ways of disseminating and collecting information from patients and caregivers.
“You have to start thinking about it from a digital perspective as we all live in a digital age and so people really do go online to search for information about their condition and about new treatment options. Making sure that you are in that space — so that if they are looking for a clinical trial or looking for more information, that they can find you — really leads to a more informed patient in the end,” says Batiste.
During the course of a trial, patients need to feel supported and comfortable. It is critical to keep patients updated with the latest information as it becomes available, which can include preliminary data and general feedback on the trial, which should come from them as well. Patients should be provided items on site to make them feel comfortable such as blankets, digital tools such as tablets/iPads, and Netflix cards, for example, to make things less burdensome and as comfortable as possible for them.
The take-home message is that patients must feel valued and supported from the beginning and be provided information that they can understand throughout the course of a trial.
According to Campbell, “it is very important to get your message out — especially in rare disease, which is a small community. Make sure you are controlling that message as much as possible by communicating with advocacy groups. [You should] not only make sure that you are driving the message, but that [patients] are aligned with the message.”
Retention Support Measures
An important component of any clinical trial is patient retention as it is important to ensure that patients are motivated to continue in a trial. Ultimately, patients should feel valued and be presented with supportive options, education and information throughout a trial. These can be achieved through site and advocacy group partnerships, pre-screening tools, patient outreach, strategic site and patient tools, among other things.
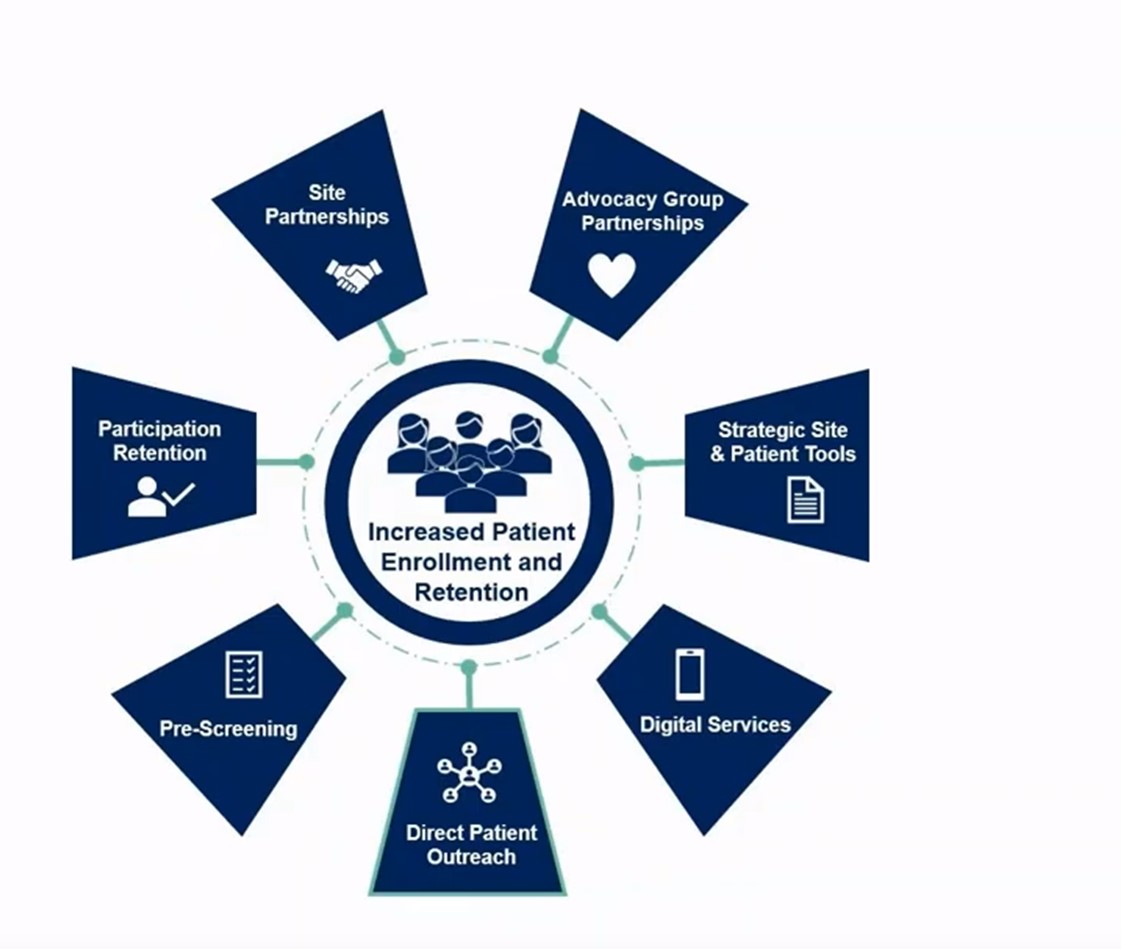
Moreover, pertinent information — such as length of the trial and possible extensions to the trial if things go well — should be clearly and openly discussed. Transparency is key in building a strong and communicative relationship with patients and their caregivers.
In the end, all of this contributes to enacting and applying robust patient-centered approaches which translates into greater compliance and successful outcomes with potential discovery of effective therapeutics that ultimately benefit the patient.
Connecting Patients to Clinical Trials
A patient-centric approach is in the best interest of everyone involved in a clinical study, particularly in trials for rare diseases. Patients with rare diseases and their caregivers represent a unique group of patients that must be educated and supported in accordance to their particular needs and goals.
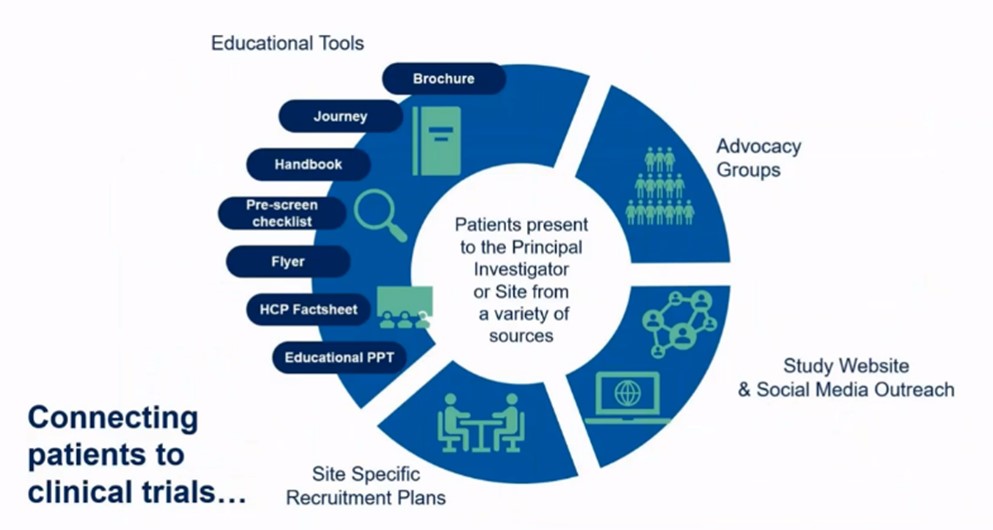
Connecting with patients and caregivers can be achieved by partnering with advocacy groups through which outreach programs and educational materials including digital tools can be relayed. Other tools include brochures, patient handbooks, pre-screen checklists, flyers and factsheets are also useful.
The engagement and inclusion of patients and advocacy groups early on can help guide strategic planning in trial development. Patient-centered approaches involving advocacy group partnerships and patient outreach significantly impact patient enrollment and retention, which serves to help achieve successful outcomes in rare disease clinical trials. Medpace and BDSRA are highly committed to helping foster and guide patient-centric approaches in rare disease clinical trials and have shown different ways of engaging patients and caregivers in this informative webinar.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.







Join or login to leave a comment
JOIN LOGIN