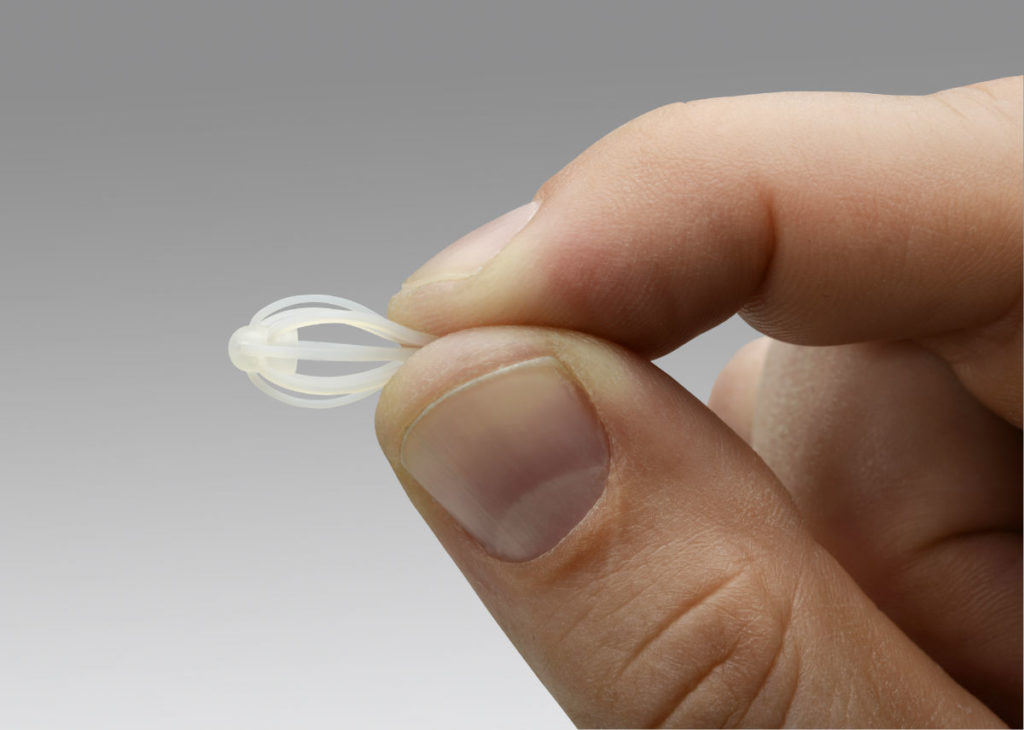
The US Food and Drug Administration (FDA) has approved Intersect ENT’s Sinuva Sinus Implant designed to treat patients with recurrent nasal polyp disease. The medical device was developed to be an additional treatment option for patients who have previously undergone ethmoid sinus surgery to remove nasal polyps.
Sinuva can be placed during a routine visit to the physician’s office, and it releases an anti-inflammatory drug treat recurrent polyps for a three-month period. In a clinical trial of Sinuva, the device was found to reduce the extent of the disease by 63 percent, compared to the control.
“Sinuva represents a much-needed breakthrough for the many nasal polyp sufferers who are seeking an effective treatment,” said Dr. Robert C. Kern, Chairman of Otolaryngology – Head and Neck Surgery at Northwestern University Feinberg School of Medicine. “For many patients struggling to manage this disease, the current treatment approaches of repeat surgeries and high-dose oral steroids have significant limitations, and intranasal sprays and rinses rely heavily on patient compliance. I look forward to offering Sinuva to my patients.”
It’s estimated that 635,000 people in the US have undergone sinus surgery to treat nasal polyps, but continue to visit their ear, nose and throat specialist to address recurring symptoms. Nasal polyps can cause a number of problems, ranging from loss of sense of smell and nasal congestion to infections and other complications.
“We are pleased that the approval of Sinuva will give patients with recurrent nasal polyps a new option,” said Lisa Earnhardt, president and CEO of Intersect ENT. “This FDA approval – our fourth commercial product, and our first product to be regulated as a pharmaceutical – is an exciting milestone for our team.”
According to Earnhardt, the company plans to launch Sinuva in the second quarter of 2018. Until then, the Intersect ENT will focus on educating physicians on the benefits of the medical device.











Join or login to leave a comment
JOIN LOGIN