Infectious disease therapies continue to play a critical role in safeguarding public health and driving commercial success. As companies navigate a complex environment shaped by emerging pathogens, evolving regulatory frameworks and shifting market demands, sales data offers valuable insights into which drugs are setting the pace and why. This blog examines the top 10 infectious disease drugs based on recent sales performance, shedding light on the factors that have driven these treatments to the forefront of the industry.
From breakthrough HIV therapies to vital vaccines addressing COVID-19, human papillomavirus (HPV) and other life-threatening infections, these products not only underscore advances in clinical efficacy but also highlight strategic market positioning. By exploring key metrics — from sales figures and pricing structures to clinical trial outcomes and regulatory milestones — this analysis provides a snapshot of how these drugs are meeting both patient needs and business objectives.
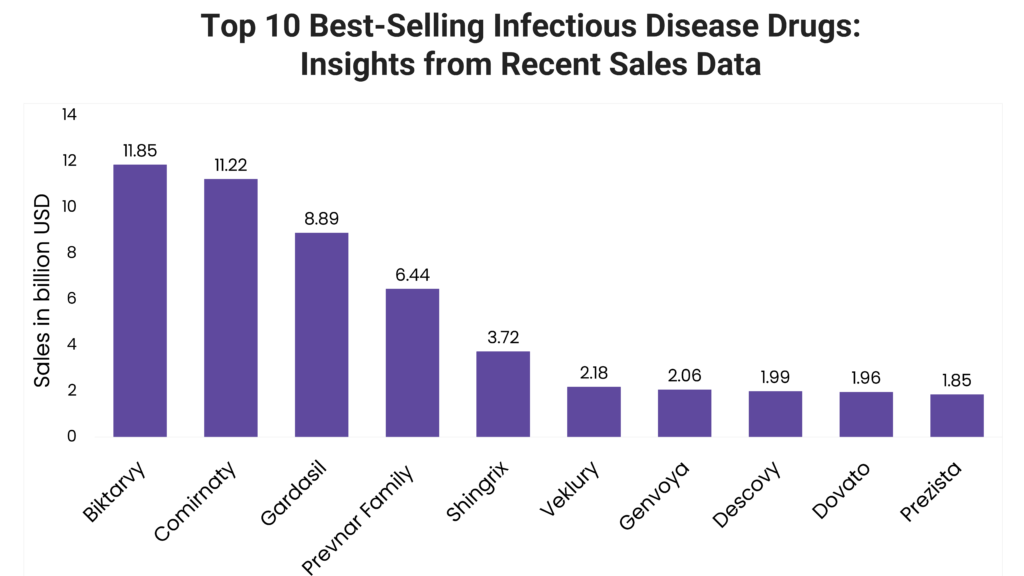
Read on to explore the leading infectious disease drugs, analyzing their sales performance from full-year 2023, market dynamics and key clinical benefits that have contributed to their success.
Note: When it comes to companies that report in foreign currencies, the conversion to US dollars uses the average annual exchange rates reported by the US Federal Reserve.
XTALKS WEBINAR: The Evolving Global AI Regulatory Landscape: Recent Developments and Practical Insights for Clinical Trials
Live and On-Demand: Thursday, March 20, 2025, at 11am EDT (4pm CET/EU-Central)
Register for this free webinar to understand the evolving AI regulatory landscape and drive innovation in clinical trials.
1. Biktarvy (Bictegravir/Emtricitabine/Tenofovir Alafenamide)
Biktarvy 2023 sales: $11.85 billion
Company/developer: Gilead Sciences
Date of first FDA approval: February 7, 2018
FDA-approved indications: HIV-1
Price of Biktarvy: The list price of Biktarvy is $4,216 per month.
Why it sold so well: Biktarvy generated $11.85 billion in sales last year, making up nearly 44% of Gilead’s total product revenue and over half of its HIV portfolio — its primary sales driver. According to Gilead’s 2023 annual report, Biktarvy experienced a 14% year-over-year (YoY) sales increase, driven by heightened demand and a higher average realized price.
In Q4 2023, Biktarvy’s sales grew by 7% to reach $3.1 billion compared to the same period in 2022 — a gain attributed to increased demand that was partially offset by a lower average price due to the channel mix.
Looking ahead, Gilead expects its HIV franchise, led by Biktarvy, to expand by 4% in full-year 2024 as demand continues to rise and the drug’s market share grows.
2. Comirnaty (COVID-19 Vaccine, mRNA)
Comirnaty 2023 sales: $11.22 billion
Company/developer: Pfizer and BioNTech
Date of first FDA approval: August 23, 2021
FDA-approved indications: Prevention of COVID-19
Cost of Comirnaty: Comirnaty intramuscular suspension (30 mcg/0.3 mL) costs about $10 for a supply of 18 mL.
Why it sold so well: Although Pfizer’s COVID-19 vaccine was initially expected to deliver a brief but record-setting impact as a potential pandemic solution, it nonetheless achieved remarkable sales. In its first full year on the market in 2021, the vaccine generated $36.8 billion — marking the highest annual revenue ever for a single pharmaceutical product. The following year saw sales edge up to $37.8 billion, outpacing Moderna’s Spikevax, which earned $36 billion over two years.
Thanks to the success of Comirnaty, Pfizer reached a record-breaking $100.33 billion in total sales for 2022, the most ever reported by any pharma company in a single year. However, as the pandemic waned in 2023, sales of the Pfizer-BioNTech vaccine fell by 70% to just over $11 billion, and overall, Pfizer’s total revenue dropped by more than 40% to $58.5 billion, driven by declining sales of both its vaccine and the COVID-19 antiviral Paxlovid.
3. Gardasil (HPV Quadrivalent Vaccine, Recombinant; HPV 9-Valent Vaccine, Recombinant)
Gardasil 2023 sales: $8.89 billion
Company/developer: Merck & Co.
Date of first FDA approval: June 8, 2006 (Gardasil); December 11, 2014 (Gardasil 9)
FDA-approved indications: Gardasil and Gardasil 9 vaccines are approved for the prevention of the following indications caused by HPV. Gardasil 9 is approved for use in individuals aged nine through 45 years while Gardasil is indicated for a similar range but focuses on a slightly younger demographic, up to 26 years old:
- Cancers: Prevents cervical, vulvar, vaginal, anal and certain head and neck cancers
- Genital warts: Caused by specific HPV types
- Precancerous lesions: Includes various grades of cervical, vulvar, vaginal and anal intraepithelial neoplasias
Life price of Gardasil 9: $286.78 per dose
Why it sold so well: Gardasil and Gardasil 9 have proven to be highly effective in preventing infections from HPV strains that lead to serious health issues, such as cancers and genital warts. These vaccines address the widespread challenge posed by HPV and its strong association with various cancers and other diseases. Notably, Gardasil 9 expands protection by covering five additional HPV types beyond those targeted by the original Gardasil, making it the only HPV vaccine approved for use in the US since 2016.
In 2023, combined sales of Gardasil and Gardasil 9 nearly reached almost $8.9 billion, marking a 29% increase from the previous year. In China, this surge was driven by expansive vaccination campaigns and heightened public awareness of HPV-related cancer prevention, while in the US, growth was spurred by public-sector procurement and increased immunization rates. When foreign exchange impacts are excluded, the sales growth for these vaccines was even more robust at 33%.
This significant growth in Gardasil sales has been crucial for Merck, helping to offset declines in other areas, such as lower sales of their antiviral drug Lagevrio (molnupiravir).
Clinical trials have shown that Gardasil 9 is exceptionally effective in preventing diseases caused by the HPV types it targets. In a recent development, Merck announced plans to initiate clinical trials for a new multi-valent HPV vaccine and to evaluate a single-dose regimen of Gardasil 9, with the goal of broadening protection against various HPV types while simplifying the vaccination schedule.
In Australia, Gardasil is part of the National Immunization Programs for adolescents aged 12 to 13, underscoring the importance of early intervention in preventing HPV-related diseases.
Additionally, under Merck’s “Merck Vaccine Patient Assistance Program,” Gardasil 9 is provided free of charge in the US to uninsured individuals aged 19 to 45 with low household incomes.
4. Prevnar Family (Pneumococcal 7-Valent Conjugate)
Prevnar Family 2023 sales: $6.44 billion
Company/developer: Pfizer
Date of first FDA approval: February 2000
FDA-approved indications: Active immunization for the prevention of pneumonia and invasive disease caused by S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F
Cost of Prevnar 20: $261.56 per dose
Why it sold so well: In 2023, the Prevnar Family generated $6.44 billion for Pfizer, representing 11% of the company’s overall revenue. The journey began in 2000 with the FDA’s approval of the first pneumococcal conjugate vaccine, PCV7 (Prevnar), which quickly became a routine immunization for children in the US, protecting against seven serotypes of pneumococcal bacteria.
In 2010, the FDA approved PCV13 for children, extending protection to six additional serotypes by stimulating the body’s production of antibodies, similar to the response seen with PCV7.
More recently, in 2021, the FDA approved PCV20 for adults aged 18 and older, and in 2023, its use was extended to children aged six weeks through 17 years — making Prevnar 20 the pediatric vaccine with the broadest serotype coverage, guarding against all 20 serotypes included in the formulation. Given the significant reduction in pneumococcal disease among children achieved through these vaccines, the WHO has recommended that PCVs be integrated into national immunization programs.
5. Shingrix (Zoster Vaccine Recombinant, Adjuvanted)
Shingrix 2023 sales: $3.72 billion
Company/developer: GlaxoSmithKline (GSK)
Date of first FDA approval: October 20, 2017
FDA-approved indications: Shingrix is a vaccine indicated for the prevention of herpes zoster (HZ; shingles).
Price of Shingrix: The shingles vaccine costs approximately $400 for the complete two-dose regimen or about $200 per dose.
Why it sold so well: In 2023, GSK’s vaccine sales grew by 25%, with the shingles vaccine Shingrix generating 3.4 billion GBP ($4.3 billion USD) in sales. A Phase III clinical trial program assessed Shingrix’s efficacy in over 38,000 participants. A pooled analysis of these studies revealed that Shingrix exhibited over 90% efficacy against shingles across all age groups and maintained this efficacy over a four-year follow-up period. Additionally, by preventing shingles, Shingrix also reduced the incidence of postherpetic neuralgia (PHN), a common and chronic complication of shingles.
Related: Top 10 Heart and Vascular Medications Based on Recent Sales Data
6. Veklury (Remdesivir)
Veklury 2023 sales: $2.18 billion
Company/developer: Gilead Sciences
Date of first FDA approval: October 22, 2020
FDA-approved indications: Veklury is indicated for the treatment of COVID-19 in adults and pediatric patients aged 12 years and older who weigh at least 40 kg and require hospitalization.
Price of Veklury: In the US, the list price for Veklury is $520 per vial.
Why it sold so well: Veklury is approved in over 50 countries globally, and to date, it, along with generic remdesivir, has been administered to nearly 13 million patients worldwide.Veklury sales fell by 28% to $720 million in the fourth quarter of 2023 compared to the same period in 2022, primarily due to reduced rates of COVID-19-related hospitalizations. Veklury sales typically correlate with the rates and severity of COVID-19 infections and hospitalizations. The clinical benefits of Veklury in hospitalized patients have been demonstrated through multiple randomized, controlled Phase III trials, including ACTT-1. In 2020, ACTT-1 results showed a trend toward reduced mortality in Veklury-treated patients compared to those receiving a placebo (11% vs. 15%), although this difference was not statistically significant.
7. Genvoya (Cobicistat, Elvitegravir, Emtricitabine and Tenofovir Alafenamide)
Genvoya 2023 sales: $2.06 billion
Company/developer: Gilead Sciences
Date of first FDA approval: November 5, 2015
FDA-approved indications: Genvoya is indicated as a complete regimen for the treatment of HIV-1 infection.
Price of Genvoya: The cost for Genvoya (150 mg-150 mg-200 mg-10 mg) oral tablet is around $4,411 for a supply of 30 tablets.
Why it sold so well: Genvoya achieved $2.06 billion in international sales in 2023. In combined clinical studies, 1,733 treatment-naïve adults with HIV were randomized to receive either Genvoya or Stribild (elvitegravir/cobicistat/emtricitabine/tenofovir). At Week 144, 84.2% of patients on Genvoya and 80% of those on Stribild achieved HIV-1 RNA levels below 50 copies/mL. Additionally, 81.1% of patients on Genvoya and 75.8% of those on Stribild achieved HIV-1 RNA levels below 20 copies/mL, which was a secondary endpoint. Genvoya shows significantly higher rates of virologic suppression and more favorable renal and bone laboratory parameters compared to Stribild.
8. Descovy (Emtricitabine and Tenofovir Alafenamide)
Descovy 2023 sales: $1.99 billion
Company/developer: Gilead Sciences
Date of first FDA approval: April 4, 2016
FDA-approved indications: Descovy is used for the treatment of HIV-1 infection. It is also approved for pre-exposure prophylaxis (PrEP) to reduce the risk of HIV-1 infection.
Price of Descovy: The list price of Descovy is $2,202 per month.
Why it sold so well: In 2023, Descovy sales rose by 6% to $2.0 billion compared to 2022, largely due to favorable channel inventory dynamics and increased demand. Clinical trial data showed that over 90% of patients receiving Descovy treatment had HIV-1 RNA less than 50 copies/mL at Week 48.
In 2019, the FDA approved Descovy for HIV-1 PrEP to reduce the risk of HIV-1 infection. The trial showed that Descovy was similar to Truvada (emtricitabine/tenofovir) in reducing the risk of acquiring HIV-1 infection.
9. Dovato (Dolutegravir and Lamivudine)
Dovato 2023 sales: $1.96 billion
Company/developer: ViiV Healthcare/GSK
Date of first FDA approval: April 8, 2019
FDA-approved indications: Dovato is indicated as a complete regimen for the treatment of HIV-1 infection.
Price of Dovato: The list price for a 30-day supply of Dovato is $3,095.69.
Why it sold so well: Dovato remains the top-selling product in GSK’s HIV portfolio, with sales of 516 million GBP ($663 million USD) in the fourth quarter of 2023. Dovato is the first FDA-approved two-drug, fixed-dose regimen for HIV-infected adults who have never received HIV treatment. Its efficacy and safety were established in two identical, randomized, double-blind, controlled clinical trials involving 1,433 HIV-infected adults with no prior antiretroviral treatment. These trials demonstrated that the regimen of dolutegravir and lamivudine effectively reduced HIV levels in the blood to less than 50 copies/mL, similar to the effect of another regimen containing dolutegravir, emtricitabine and tenofovir, with patients maintaining low HIV RNA levels for at least 48 weeks.
10. Prezista (Darunavir/Cobicistat)
Prezista 2023 sales: $1.85 billion
Company/developer: Janssen Pharmaceuticals
Date of first FDA approval: June 23, 2006
FDA-approved indications: Prezista is indicated for the treatment of HIV-1 infection. It must be co-administered with ritonavir and used in combination with other antiretroviral agents.
Price of Prezista: The cost of Prezista (800 mg oral tablet) is about $2,262.36 for a supply of 30 tablets.
Why it sold so well: The efficacy of Prezista/ritonavir is supported by a 192-week analysis from a randomized, controlled, open-label Phase III trial. Patients receiving Prezista/ritonavir demonstrated a confirmed virologic response of less than 50 copies/mL at Week 48, with 81% maintaining undetectable levels at Week 192, compared to 68% with lopinavir/ritonavir.
If you want to have your organization featured on Xtalks, please email Vera Kovacevic, PhD, at: [email protected]











Join or login to leave a comment
JOIN LOGIN