Emmaus Life Sciences’ sickle cell disease drug Endari (L-glutamine oral powder) has been approved by the US Food and Drug Administration (FDA). Patients age five years and older will be eligible for treatment with Endari to reduce severe complications associated with the inherited blood disorder.
“Endari is the first treatment approved for patients with sickle cell disease in almost 20 years,” said Dr. Richard Pazdur, acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research and director of the FDA’s Oncology Center of Excellence. “Until now, only one other drug was approved for patients living with this serious, debilitating condition.”
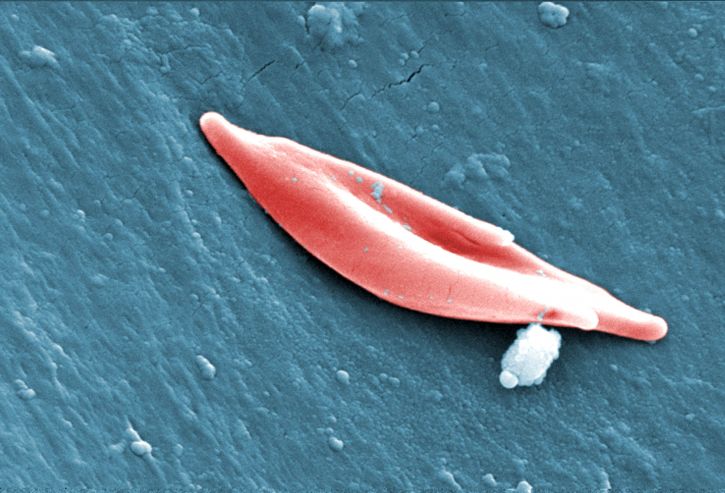
Patients with sickle cell disease produce abnormally-shaped red blood cells, which limits their oxygen carrying capacity and the ability of these cells to travel through blood vessels. This condition results in decreased oxygen delivery to tissues around the body, leading to organ damage and severe pain.
Around 100,000 individuals in the US – mostly African-Americans and Latinos – suffer from sickle cell disease, according to the National Institutes of Health. Patients with sickle cell disease have an average life expectancy of 40 to 60 years.
A randomized clinical trial involving sickle cell disease patients ages five to 58 years old was conducted to determine the safety and efficacy of Endari. Patients were enrolled in the trial based on the criterion that they had experienced two or more painful crises within the past 12 months.
“A sickle cell crisis is the most common acute complication for patients and the number one cause of emergency room visits,” said Dr. Wally Smith, Florence Neal Cooper Smith Professor of Sickle Cell Disease, Division of General Internal Medicine, Virginia Commonwealth University. “Endari has clinically shown to reduce sickle cell crises and hospitalizations, representing a significant medical advancement for patients with limited therapeutic options that have many side effects.”
Compared to patients given a placebo, those that were treated with Endari experienced fewer hospital visits for pain episodes. In addition, these patients showed a reduced incidence of acute chest syndrome – a potentially life-threatening complication of sickle cell disease – compared with the placebo group.
“The approval of Endari is a significant milestone for the sickle cell patient community who has not had an advancement in treatment for nearly 20 years and which now, for the first time ever, has a treatment option for children,” said Dr. Yutaka Niihara, Chairman and Chief Executive Officer of Emmaus Life Sciences. “Endari reinforces our commitment to discovering innovative therapies that help to improve the lives of people with rare diseases.”
Emmaus was originally granted Orphan Drug Designation by the FDA for Endari. The company plans to make the treatment available to sickle cell disease patients as early as the fourth quarter of 2017.










Join or login to leave a comment
JOIN LOGIN