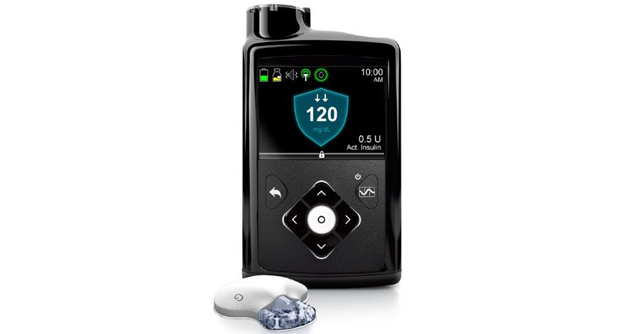
Medical device company Medtronic is sponsoring a large, multicenter clinical trial across the US aimed at helping individuals with type 1 diabetes better manage their condition. The study will feature the company’s MiniMed 670G Insulin Pump System, an artificial pancreas device designed to continuously monitor blood glucose and automatically deliver insulin doses when needed.
For the three million Americans diagnosed with type 1 diabetes – a number that’s growing by 15,000 new cases each year – regular glucose monitoring and insulin injection are a burden on everyday life. This constant disease management is required to reduce a patient’s risk of diabetes-related health complications, including nerve damage, kidney disease and eye disease.
“Type 1 diabetes is a very serious and challenging disease for those affected,” said Dr. Rodica Pop-Busui, professor of internal medicine in the Michigan Medicine Division of Metabolism, Endocrinology and Diabetes. “In spite of important discoveries in patient care and prevention of complications, living daily with type 1 diabetes continues to be a struggle for many patients.”
Medtronic’s trial will be the largest of its kind to date. The company’s hybrid closed loop system was approved by the FDA in 2016 as the first artificial pancreas on the market.
“The system monitors blood glucose levels and insulin delivery continuously and adjusts automatically the dose of insulin, helps the patient maintain stable blood sugar levels throughout the day and night and enables personalized and automated delivery of basal insulin,” said Pop-Busui, the primary investigator of Michigan Medicine’s trial site. “Thus, by acting like an artificial pancreas, it is bypassing many of the patients’ frequent insulin dose calculations and alleviating some of the burden associated with daily diabetes management, which can be strenuous and wearing.”
The clinical trial is actively enrolling up to 1,500 patients ages two to 80 with type 1 diabetes. Patients will be asked to use Medtronic’s MiniMed 670G system to monitor their blood glucose at home, which requires regular entry of carbohydrate consumption, periodic sensor calibration and acceptance of bolus correction recommendations.
The large trial will help Medtronic assess the safety and efficacy of its artificial pancreas device in the target population.
“We hope to help patients across all ages with type 1 diabetes reach better glucose control with lower risk for hypoglycemia and better quality of life,” said Pop-Busui. “We are working together, adult and pediatric endocrinologist physicians and nurse investigators, in a concerted effort to fight diabetes and its complications.”











Join or login to leave a comment
JOIN LOGIN