Bayer, a renowned German pharmaceutical company, has recently announced that its Ultravist 300 and 370 (iopromide) injection has won approval from the US Food and Drug Administration (FDA) for use in contrast-enhanced mammography (CEM). This approval distinguishes Ultravist as the singular contrast agent in the US specifically indicated for visualizing confirmed or suspected breast lesions in adults in conjunction with mammography and/or ultrasound.
Ultravist (iopromide) injection is available in two strengths for CEM: Ultravist 300 (300 mg iodine per mL) and Ultravist 370 (370 mg iodine per mL).
“The approval of Ultravist-300 and -370 in contrast-enhanced mammography gives physicians a new imaging option where conventional mammography might not be enough,” said Dr. Konstanze Diefenbach, head of Radiology Research and Development at Bayer, in the company’s news release. “We are pleased to be able to offer additional options for breast imaging to healthcare professionals, as we aim to support them in their role of providing clear answers from diagnosis to care for patients.”
The FDA’s approval of Ultravist further enhances Bayer’s comprehensive breast imaging portfolio, which already includes Gadavist (gadobutrol) injection (a gadolinium-based contrast agent approved for magnetic resonance imaging [MRI]) and the Medrad Stellant Flex computed tomography (CT) injection system with the Certegra workstation for use in CEM.
XTALKS WEBINAR: Innovating Oncology Trials: Breaking Access Barriers, Optimizing Protocols and Navigating Regulatory Challenges
Live and On-Demand: Tuesday, July 11, 2023, at 10am EDT (4pm CEST/EU-Central)
Register for this free webinar to gain valuable insights optimizing trial design, into overcoming barriers and adhering to the evolving regulatory landscape. Ultimately, this knowledge will contribute to equitable access to innovative treatments, improved trial efficiency and enhanced patient outcomes.
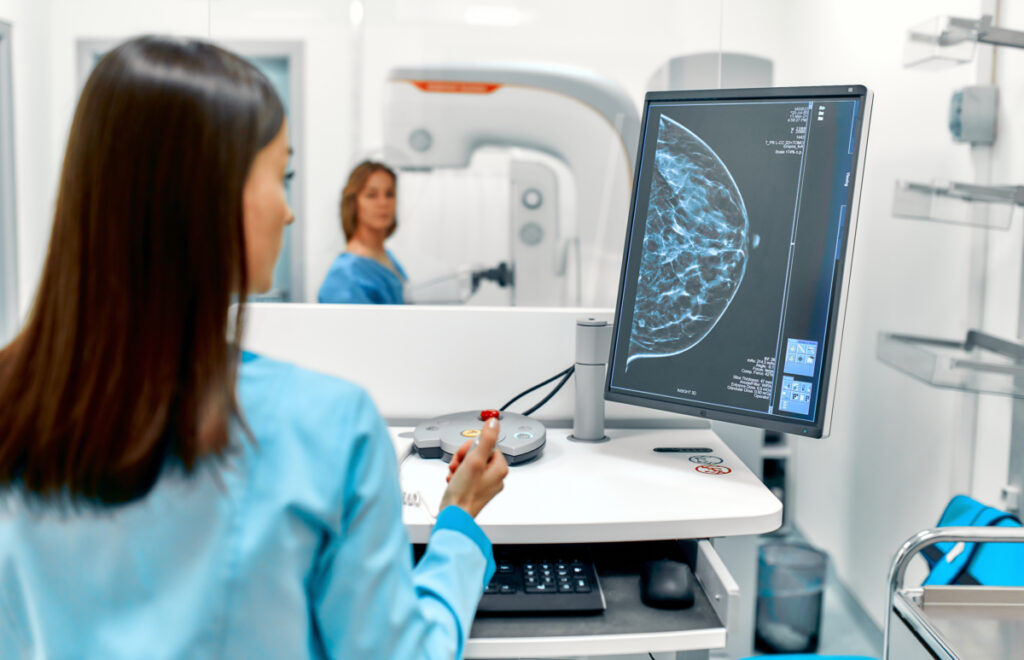
What is Contrast-Enhanced Mammography (CEM)?
Contrast-enhanced mammography (CEM) is a revolutionary imaging technique that uses iodinated contrast materials to augment the visualization of neovascularity in breast tissue, analogous to MRI. By implementing a dual-energy approach, CEM capitalizes on the inherent contrast in X-ray attenuation between breast tissue and iodine, thereby accentuating areas within the breast where the contrast agent is absorbed. These zones of contrast agent uptake often correlate with breast cancer.
Breast cancer is associated with tumoral angiogenesis, a process marked by the compromised integrity of the basement membrane, which allows the vessel wall to become permeable to a contrast agent, leading to enhancement. CEM leverages this correlation to pinpoint and illuminate areas of potential concern, assisting in the detection and diagnosis of breast cancer.
How Does Ultravist (iopromide) Work?
Ultravist (iopromide) is a non-ionic radiographic contrast agent used in multiple indications for various imaging procedures.
For intra-arterial procedures, it is employed for cerebral arteriography, peripheral arteriography, coronary arteriography, left ventriculography, visceral angiography, aortography and radiographic evaluation of cardiac chambers in pediatric patients aged two years and older.
In intravenous procedures, Ultravist is used for excretory urography, contrast CT of the head and body and CEM in adults as an adjunct to mammography and/or ultrasound.
It’s noteworthy that specific concentrations and presentations of Ultravist are recommended for each type of imaging procedure.
Ultravist is not approved for intrathecal use because intrathecal administration (even if inadvertent) of Ultravist may cause death or other harm.
Other Iodine-Based Contrast Agents
There are numerous FDA-approved iodine-based contrast agents available for medical imaging purposes. Recently, Bracco Diagnostics, a US company specializing in diagnostic imaging, procured FDA import discretion for Iomeron (iomeprol injection) to address the shortage of iodinated contrast media. The FDA has not approved Iomeron. Iomeron is a non-ionic iodinated contrast medium designed for adult intravascular use in a variety of imaging procedures. It is currently registered in over 50 countries across Europe and Asia.
Moreover, Fresenius Kabi, a global healthcare company based in Germany, introduced a range of generic contrast media agents in the US in 2022. They promptly launched Iodixanol Injection, USP, an FDA-approved generic iodinated contrast media agent used in diagnostic X-ray-based imaging procedures such as CT scans. This generic option offers hospitals and clinics an alternative for diagnosing certain disorders of the brain, blood vessels, heart, kidneys and other internal organs.











Join or login to leave a comment
JOIN LOGIN