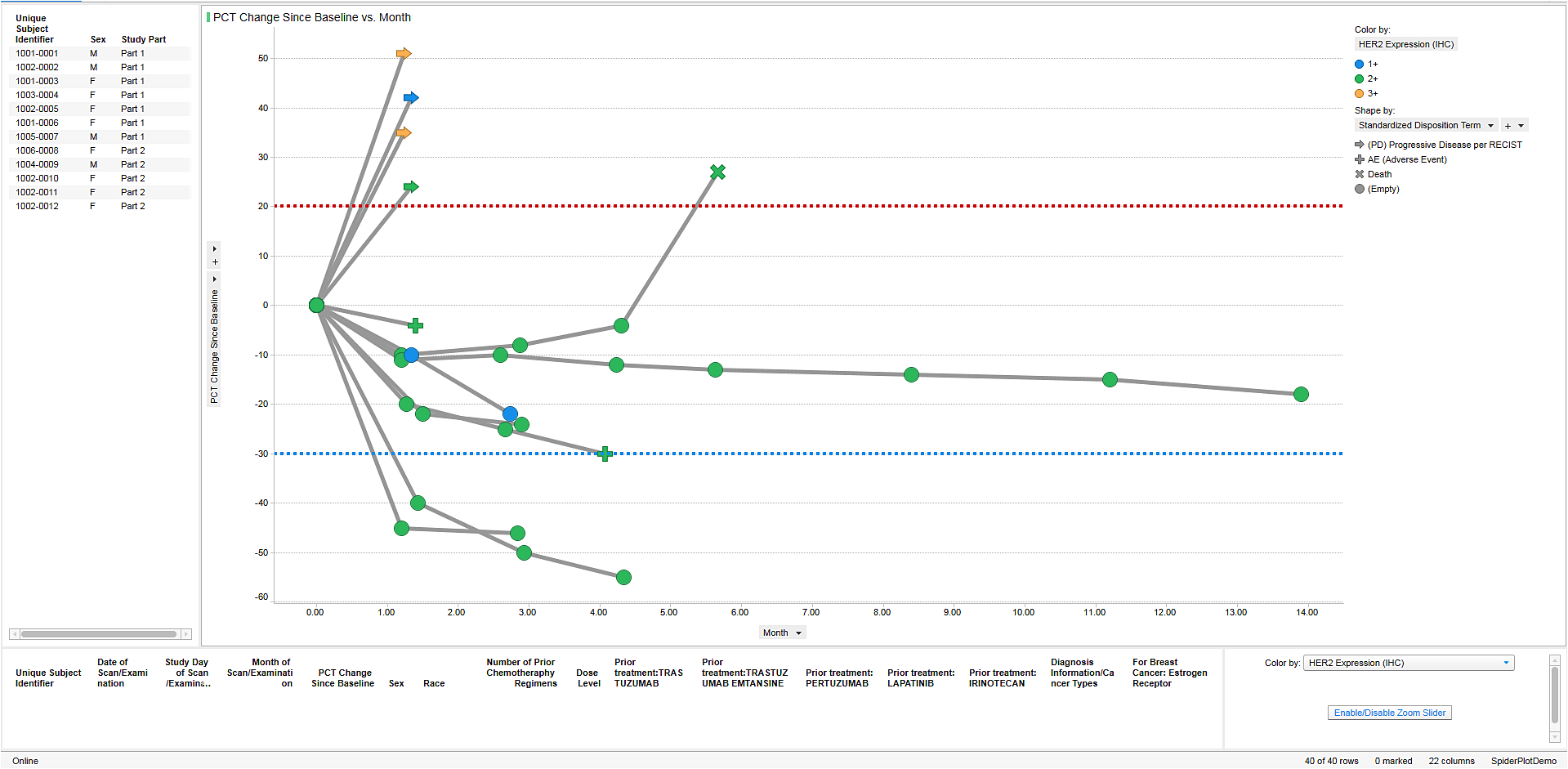
The industry is obsessed with speed and flexibility, and rightly so. Nothing exemplifies these characteristics, like the global race for COVID-19 interventions. PerkinElmer’s TIBCO Spotfire solutions have been at the forefront of clinical data visualization and analysis. The company is proud that its products are contributing to developing therapies and vaccines to address this viral pandemic.
This webinar will highlight how many of the challenges our clients experience during clinical trials can be tackled. Analytics can be tailored for specific clinical studies and therapeutic areas. With deep Spotfire, domain expertise and proven industry success, PerkinElmer wants to share the secret sauce on fast and flexible clinical data analysis.
Speakers

Brent Meyers, Director, Clinical & Translational Analytics, PerkinElmer
Brent Meyers has been building analytics solutions and leading technical delivery teams for 17 years. While he has focused on clinical analytics for the majority of his career, Meyers has also worked in the defense industry and US navy. He leads each analytics challenge with a focus on design and leveraging the best talent and technology for the solution. Meyers holds a BA from The Citadel and an MBA from Meredith College.

James Bullis, Sr. Software Service Specialist, PerkinElmer
James Bullis has spent the last eight years in the field of Biomedical Informatics. During that time, he has worked for and with Pharmaceutical and Biotechnology companies in almost every aspect of drug development, from Pre-Clinical Discovery to Clinical Trials. He received his PhD from the University of Washington, Seattle and conducted postdoctoral work at Brandeis University.
Who Should Attend?
- Clinical Safety, Director / VP
- Clinical Operations Director / VP
- Clinical Research & Development Director / VP
- Pharmacovigilance / Drug Safety Officer
- Clinical Pharmacology
- Quantitative Medicine
- Clinical Data Sciences
- Biostatistics and Data Management Director / VP
- Clinical / Medical Monitoring
- Clinical or R&D IT, Director or VP
What You Will Learn
In this webinar, attendees will learn how to:
- Accelerate your clinical data review
- Review safety and efficacy data in a centralized location
- Leverage an extensive library of therapeutic analytics
- Effectively perform an in-stream review of study data, to make smarter decisions faster
Xtalks Partner
PerkinElmer
PerkinElmer’s advanced analytics and services solutions for Clinical Development help the world’s leading biopharmaceutical, medical device and diagnostics manufacturers discover new therapeutics faster by streamlining clinical operations, transforming risk into safety and enabling actionable decisions that can lead to better health outcomes.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account