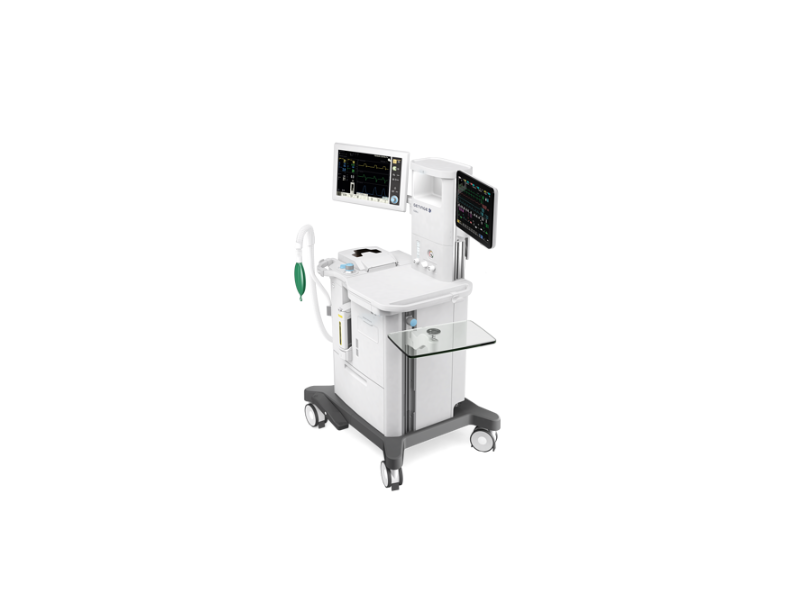
Among Getinge’s line-up of anesthesia systems, the Flow-c is a compact model built for crowded or busy hospital operating rooms and ambulatory surgery centers (picture shown). Photo courtesy of Getinge USA Sales Inc.
Anesthesia systems or anesthetic machines are a system comprised of a mechanical ventilator, suction equipment, breathing system and monitoring devices. This machine delivers a mixture of medical gases and inhaled anesthetics to the patient. Only trained anesthesiologists and anesthetists in hospital settings can operate these systems.
In a new press release, the US Food and Drug Administration (FDA) identified a Class I recall (the most serious type of recall where products or devices can cause serious injuries or death) of Getinge USA Sales Inc’s Flow-c and Flow-e Anesthesia Systems after Getinge received reports of cracked or broken on/off switches on the system’s suction unit. If this switch breaks, the suction unit will not be able to remove phlegm, blood, or stomach contents from the patient’s mouth and/or airways.
Malfunctioning of the suction unit can delay procedures unless the anesthesiologist can find an alternative. An extended delay can cause the fluids to block the patient’s breathing that can result in choking, lung infection (pneumonia), acute respiratory failure or acute respiratory distress syndrome (ARDS) which causes low blood oxygen, brain injury due to low oxygen supply and death.
As of that press release, Getinge received 21 complaints, no injuries and no deaths associated with use of the devices.
This recall affects healthcare personnel who use these systems and patients who are treated in healthcare settings with these anesthesia systems.
The Flow-c and Flow-e Systems
The Flow-c and Flow-e systems were developed for patients who range from newborns to adults. They provide a streamlined workflow and feature several options for personalization and increased functionality. The systems deliver inhalation anesthesia and provide ventilation control to patients that don’t have the ability to breathe or need support for their limited breathing ability.
The anesthesia machines had received FDA 510(k) clearance in 2020 as personalized anesthesia delivery systems. “Every detail of this machine has been designed in collaboration with clinicians to ensure optimal care with high efficiency,” said Lena Evander, director of Product Management Anesthesia at Getinge in the press release statement from 2020.
How do These Anesthesia Systems Work?
The Flow-c is a compact and cost-efficient model made for crowed operating rooms and busy ambulatory surgery centers. The Flow-e supports an extended worktop, more storage, up to two anesthetic agent options and several mounting possibilities for auxiliary equipment. Both systems feature an automated gas control (AGC) that allows the operator to pre-set target inspired oxygen, speed and end-tidal anesthetic levels using an intuitive interface. An in-built O2Guard automatically increases oxygen flow should the percentage drop below 21, thus reducing the risk of hypoxia.
In anesthesia, there is time delay in the anesthetic agent concentrations between the lungs and the brain. The Flow-c and Flow-e systems are driven by the MAC Brain — a unique Getinge tool that allows the operator to visualize and control the consumption of agent concentrations. This prevents the risk of over-dosing or under-dosing. In addition, the system is equipped with lung recruitment maneuvers that allows the operator to monitor the patient’s breathing in real-time, giving critical information that could guard against atelectasis (partial or total lung-collapse).
Lastly, all Getinge Flow anesthesia machines are built with their innovative Flow Core Anesthesia Technology and Servo ICU Ventilation platform that provide improved anesthetic control and air flow accuracy with the patient’s every breath.
What to do with an Affected Flow-c and Flow-e System?
In a Medical Device Correction letter sent to risk managers by Getinge, they recommended all customers to:
- Check equipment inventory immediately to see if any affected Flow-c and/or Flow-e systems are being used
- Continue using any existing affected Flow-c and/or Flow-e systems but perform daily system and pre-anesthesia check out procedures including checking the functionality of the suction unit
- If the affected device has a cracked or broken on/off switch:
- Ensure that a temporary alternative suction substitute is available, or
- Replace the affected device with a fully functioning unit
All customers should complete and email the Medical Device Correction Response Form to Getinge [email protected] or fax to (877) 548-4901, even if they don’t possess an affected device. Affected customers will be contacted by Getinge service representatives to schedule on-site correction of the device.










Join or login to leave a comment
JOIN LOGIN