European AI med tech company AZmed received two new FDA clearances for its AI-powered chest X-ray tool, AZchest.
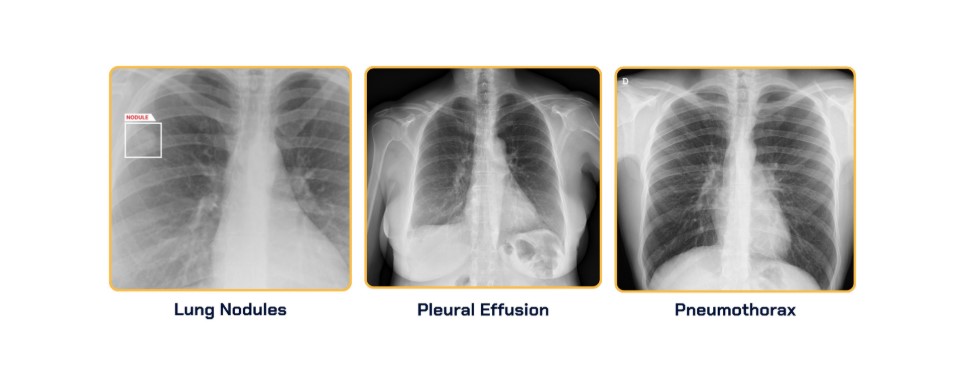
The FDA approved AZchest applications to detect lung nodules and triage cases involving pneumothorax and pleural effusion. The applications offer radiologists improved interpretation and detection of these chest X-ray abnormalities.
AZchest, a clinically validated AI radiology solution, automatically identifies, classifies and generates reports on key cardiac and pulmonary abnormalities in chest X-rays.
In a standalone study, AZchest demonstrated an 88.47% sensitivity and an 82.94% specificity in detecting lung nodules.
When experienced radiologists used AZchest in a controlled multi-reader multi-case trial, sensitivity increased by 10% to 89.35%, and the area under the receiver operating characteristic curve (AUC) improved by 5%.
For pneumothorax detection, AZchest achieved a sensitivity of 93.79% and an AUC of 98.57%.
Additionally, for pleural effusion, it achieved a sensitivity of 91.34% and an AUC of 98.30%.
Related: FDA Clears New Module for Anterion Eye Imaging Platform
AZChest’s Market Impact
The results position AZchest among the most advanced AI tools currently available in the US.
The tool aims to deliver measurable diagnostic benefits in real-world clinical settings and aid in the early detection of cancer.
Therefore, AZmed’s goal is to enhance excellence in medical imaging while emphasizing AZchest’s potential to alleviate the workload on radiologists.
Julien Vidal, CEO of AZmed, emphasized the significance of the FDA clearances, stating, “Our deep learning algorithms are designed to rapidly and accurately detect abnormalities, thereby ensuring that critical cases are flagged for clinical review promptly.”
The company rebranded the name of its AI suite from Rayvolve to AZchest.
The tool enhances X-ray diagnostics to improve accuracy and save time for healthcare professionals.
It features FDA-cleared and CE-marked solutions designed to enhance radiology workflows by prioritizing urgent cases and accelerating treatment planning.
With the addition of these FDA clearances, AZchest joins AZtrauma in the company’s expanding portfolio of AI tools authorized for use in the US.
Markedly, AZmed’s AI solutions are in use in over 2,500 healthcare centers across 55 countries.
In 2023, the US medical imaging market was valued at approximately $10.53 billion and is projected to grow at a compound annual growth rate (CAGR) of 5.1% from 2024 to 2032.
Recent advancements in chest X-ray technology focus on enhancing diagnostic accuracy and efficiency, particularly through the integration of AI.











Join or login to leave a comment
JOIN LOGIN