Boston Scientific’s Vercise Neural Navigator 5 Software for deep brain stimulation (DBS) has been granted approval by the US Food and Drug Administration (FDA).
The Vercise Neural Navigator software is used to program Boston Scientific’s Vercise DBS devices for the treatment of Parkinson’s disease and essential tremor.
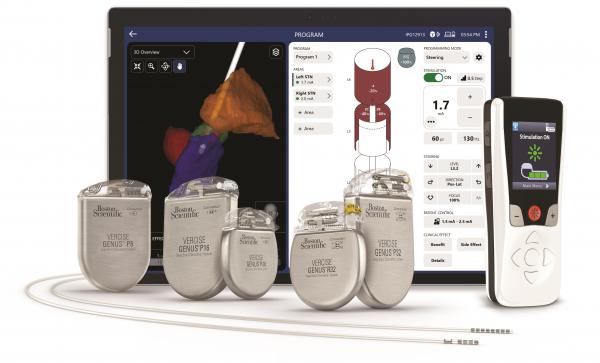
The fifth iteration of the software is designed to be specifically used with the company’s Vercise Genus devices.
DBS is used to treat the symptoms of movement disorders such as Parkinson’s and other neurological disorders. It involves neurosurgical implantation of electrodes, or leads, in affected areas of the brain, which are then connected to a small neurostimulator(s) implanted under the collarbone. The neurostimulator delivers pulses of electric current to stimulate the brain.
The fourth generation Vercise Genus DBS system delivers targeted electrical stimulation in the brain for treating Parkinson’s disease and essential tremor symptoms.
Clinicians can use Neural Navigator to configure the devices’ leads and test and set their stimulation settings.
XTALKS WEBINAR: Risk Detection in Neuroscience Trials: Are You Missing Early Warning Signs?
Live and On-Demand: Thursday, July 20, 2023, at 11am EDT (4pm BST/UK)
Register for this free webinar to explore the key factors contributing to the failure of neuroscience trials and learn effective risk detection strategies to enhance success rates.
In a press release announcing the FDA clearance, Boston Scientific said Vercise Neural Navigator 5 Software features an enhanced user interface that displays patient data in a simplified format.
It also allows clinicians to access advanced neurostimulation settings to more effectively deliver therapy and offers flexibility in treating and managing changing patient needs.
“The ability to see the precise placement of DBS Systems enables us to target therapy to meet individual needs,” said Mustafa Saad Siddiqui, medical director of the DBS program at Atrium Health Wake Forest Baptist, in the announcement. “The new features in the Vercise Neural Navigator 5 are expected to help further reduce the time needed to adjust stimulation and minimize potential side effects, allowing us to optimize treatment benefits for each patient.”
Related: World Parkinson’s Day 2023 and Promising Parkinson’s Disease Treatments
The Neural Navigator 5 software features Boston Scientific’s Stimview XT technology, which received FDA clearance in April 2022 and is the latest addition in the company’s integrated portfolio of image-guided programming solutions for its DBS system. Stimview XT generates 3D images of a patient’s anatomy to help guide the placement of the Vercise Genus DBS systems.
Developed in collaboration with software-driven med tech company Brainlab AG, Boston Scientific said that in a study it conducted, the Neural Navigator and Stimview technologies allowed clinicians to program neurostimulation settings within just 20 minutes, a reduction of 56 percent compared with standard methods. The tools also deliver real-time visualization and stimulation of an individual’s brain anatomy.
“Developing meaningful tools to help physicians provide personalized treatments for their patients delivers on our promise to advance our technologies for people living with neurological conditions,” said Boston Scientific Neuromodulation president Jim Cassidy.
“Providing effective DBS therapy is complex and can be time-consuming. This software will help streamline the process and allow for more doctor-patient interaction time.”











Join or login to leave a comment
JOIN LOGIN