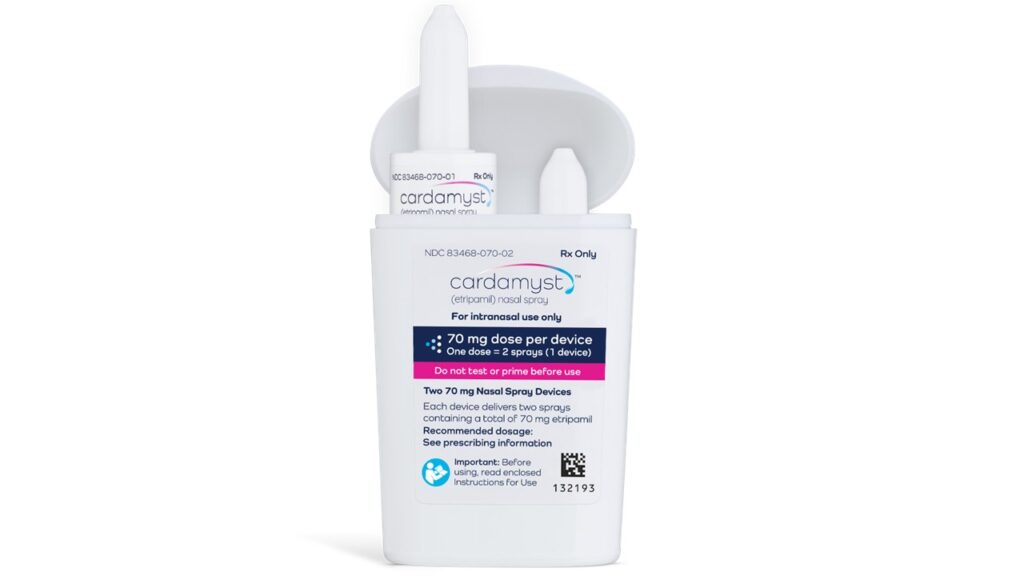
The FDA has approved Milestone Pharmaceuticals’ Cardamyst (etripamil) nasal spray, a novel prescription medication for adults experiencing acute episodes of paroxysmal supraventricular tachycardia (PSVT), a form of abnormally fast heart rhythm.
The FDA green light marks the first product approval for Montreal, Canada-based Milestone, established 22 years ago.
PSVT is a type of arrhythmia characterized by sudden, rapid heartbeats, beating over 100 beats per minute, that begin and end abruptly. Episodes can cause palpitations, lightheadedness, shortness of breath, chest discomfort and anxiety, often prompting emergency department visits. Until now, treatment options have been limited and typically required medical supervision.
PSVT happens when an electrical “short circuit” in the heart’s upper chambers causes the heart to beat too fast. Symptoms last until the heart returns to a normal rhythm, and in rare cases, repeated episodes can damage the heart and lead to dilated cardiomyopathy.
Cardamyst represents the first and only self-administered nasal spray approved by the FDA for rapid conversion of acute PSVT to normal sinus rhythm in adults.
The drug is expected to be available in US retail pharmacies in early 2026.
Related: LUMA Vision’s Verafeye Scores FDA Nod for 4D Heart Imaging Powerhouse
“Cardamyst is a novel at-the-ready treatment option that addresses the unpredictable impact of PSVT by offering patients the freedom to manage episodes anytime and anywhere,” said Joseph Oliveto, President and Chief Executive Officer of Milestone Pharmaceuticals, in a company statement. “The FDA approval of Cardamyst is a watershed moment for Milestone and a gratifying event for our team members, patients, clinical investigators, and health care providers who participated in the development program, all of whom I sincerely thank for their dedication, counsel, and collaboration toward this important achievement.”
Delivered intranasally, etripamil is a calcium channel blocker that works by reducing calcium influx in the heart’s atrioventricular (AV) node, interrupting the reentrant electrical circuits responsible for PSVT and helping restore normal rhythm.
Unlike traditional treatments that often require intravenous medications in hospital settings, Cardamyst can be used on the spot or at home, giving patients greater control and the ability to manage sudden episodes without immediate medical intervention.
Current treatments for PSVT are limited to preventive therapies, including oral beta blockers, anti-arrhythmic drugs and other calcium channel blockers, which have shown mixed effectiveness in reducing episodes.
The calcium channel blocker allows patients to avoid a visit to the emergency room, where patients receive an intravenous dose of a drug that “basically reboots your heart,” Milestone CEO Joe Oliveto said in an interview, cited by Fierce Pharma.
“It’s only a few seconds, but nevertheless, these people really don’t like it,” Oliveto explained. He said patients have told him that the IV treatment is “like a near-death experience.”
The FDA approval was supported by robust clinical trial data, notably from the Phase III Rapid study, a randomized, placebo-controlled trial enrolling hundreds of patients with PSVT.
In the study, 64% of patients who self-administered Cardamyst converted to normal sinus rhythm within 30 minutes, compared with 31% receiving placebo.
The median time to conversion was approximately 17 minutes with Cardamyst versus 54 minutes with placebo.
These results demonstrate not only significantly faster restoration of normal heart rhythm but also a reduced need for emergency care during unpredictable PSVT episodes.
Cardamyst was generally well tolerated in clinical studies. The most commonly reported side effects are mild and localized to the nasal administration site, including nasal discomfort or congestion, runny nose, throat irritation and epistaxis (nosebleeds).
Patients with certain underlying heart conditions or a history of fainting may be at higher risk for dizziness or fainting due to effects on blood pressure and heart rate, and should use the spray while seated.
Cardamyst’s approval marks the first new FDA-approved treatment for PSVT in more than 30 years and is expected to benefit more than 2 million American adults living with the condition.
Milestone Pharmaceuticals and clinical researchers are also exploring the potential for etripamil to treat other arrhythmias, such as atrial fibrillation with rapid ventricular response (AFib-RVR), representing a wider market of 10 million to 12 million Americans. If successful in future trials, patients could see additional expanded uses for the self-administered therapy.
“PSVT opens everything up for us to be transformative. It transforms us from a development company into a commercial company,” Oliveto said. “It transforms us from a single indication to now starting the AFIB Phase III indication.”
Oliveto believes one more successful Phase III trial could lead to a label expansion.
The company plans to price Cardamyst at $1,649 per prescription, a figure that translates to net revenues of approximately $500 to $1,000 per prescription for Milestone, according to chief commercial officer Lorenz Muller, who spoke during a webcast on Monday.










Join or login to leave a comment
JOIN LOGIN