The US Food and Drug Administration (FDA) has approved an electrical nerve stimulator designed to reduce the symptoms associated with opioid withdrawal. The NSS-2 Bridge medical device was developed by Indiana medical technology manufacturer Innovative Health Solutions, and was approved through the regulators de novo premarket review pathway.
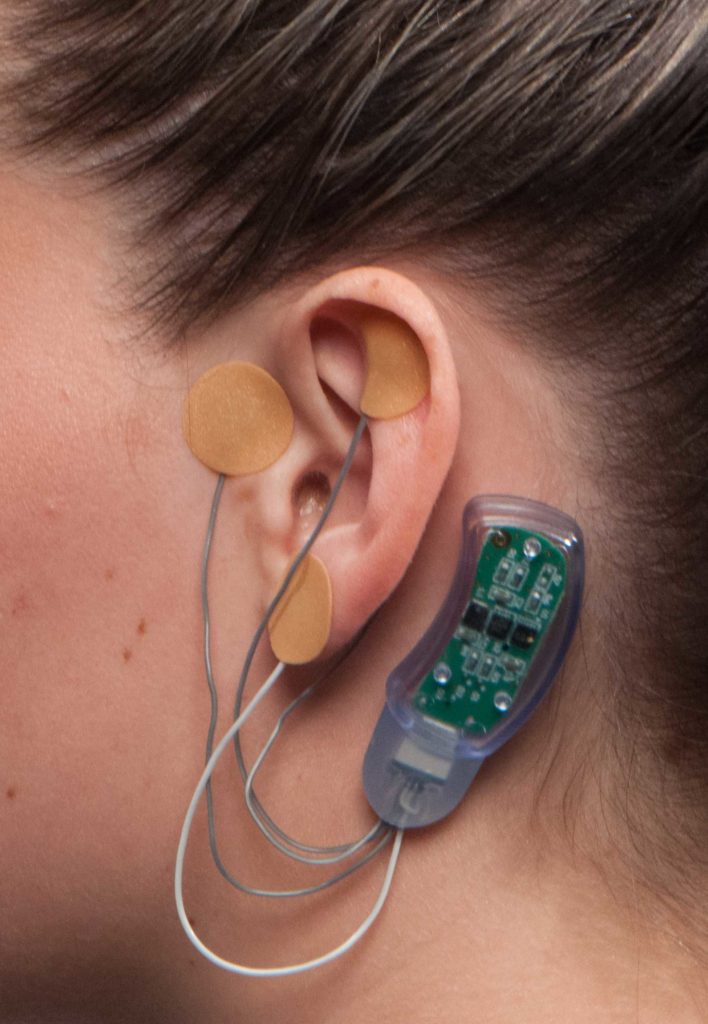
The device is designed to be placed on the skin behind a patient’s ear where it emits electric signals designed to stimulate nerves in the skull. These stimulations could help lessen the physical withdrawal symptoms patients experience when they cease to use opioids.
Sweating, insomnia, agitation, joint pain and gastrointestinal issues are all characteristic symptoms of withdrawal. The NSS-2 Bridge device can be worn for up to five days, at which point many patients will transition to medication assisted therapy.
“Given the scope of the epidemic of opioid addiction, we need to find innovative new ways to help those currently addicted live lives of sobriety with the assistance of medically assisted treatment,” said FDA Commissioner Scott Gottlieb. “There are three approved drugs for helping treat opioid addiction. While we continue to pursue better medicines for the treatment of opioid use disorder, we also need to look to devices that can assist in this therapy.”
Results from a 73-patient study were used to support the FDA’s decision to approve the medical device. Using the clinical opiate withdrawal scale (COWS) score – a measure from 0 to more than 36 of the severity of a patient’s withdrawal symptoms – the investigators of the study assessed the effectiveness of the NSS-2 Bridge device.
The average baseline COW score for patient involved in this study was 20.1, but within 30 minutes of using the electrical nerve stimulator, patients showed a reduction in the measure of at least 31 percent. The majority of the participants (88 percent) received medication assisted therapy after using the device for five days.
The NSS-2 Bridge device was originally approved by the FDA in 2014 with an indication in acupuncture. Patients detoxifying from opioids must receive a prescription from their doctor in order to use the device to manage their symptoms of withdrawal.











Join or login to leave a comment
JOIN LOGIN