Can you imagine having a day-to-day use earbud that can double as a hearing aid? The first over-the-counter (OTC) hearing aid software device, that works with compatible versions of Apple’s AirPods Pro, has been authorized by the US Food and Drug Administration (FDA), marking a breakthrough in hearing health technology. This seamless integration of hearing health into familiar technology makes the process more discreet, convenient and cost-effective, likely also reducing the stigma often associated with wearing traditional hearing aids. Apple’s commitment to blending health technology with user convenience continues to set the stage in the consumer tech space.
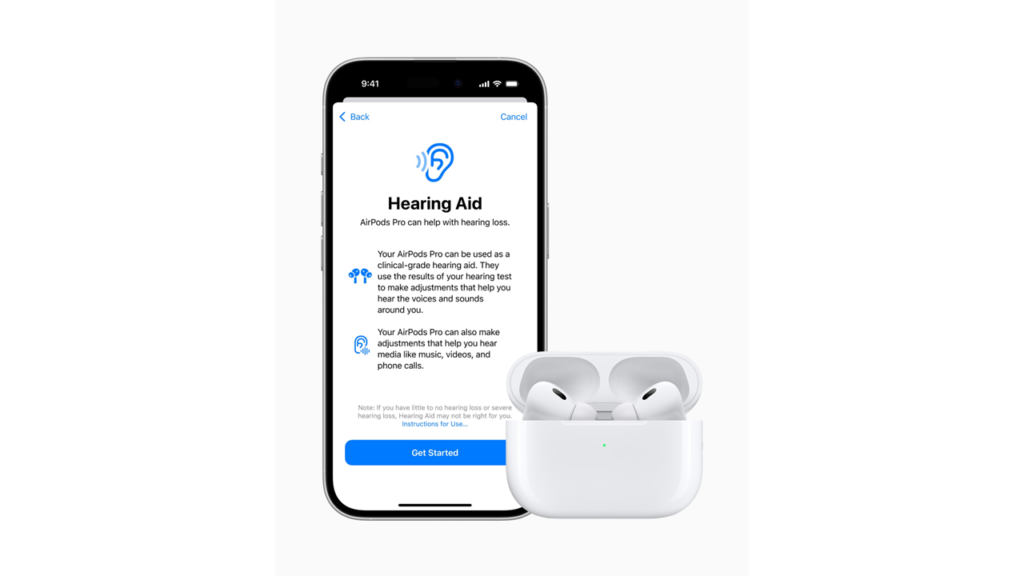
The new Hearing Aid Feature allows AirPods Pro users to convert their earbuds into clinical-grade hearing aids for individuals with mild to moderate hearing loss. This software-based solution, designed for adults 18 years and older, offers a more accessible and affordable option for hearing assistance.
This also means consumers no longer need to switch between devices for entertainment and hearing support.
Related: Oticon Medical’s Sentio System Is FDA-Cleared
Apple has long led the integration of health and technology, and this feature builds on the company’s existing hearing health tools, such as the Hearing Test and Hearing Protection. The Hearing Test is a clinical-grade self-assessment that delivers an audiogram to help calibrate the AirPods Pro according to the user’s specific needs. Meanwhile, Hearing Protection continuously tracks environmental noise and alerts users when sound levels surpass safe thresholds.
In addition to its affordability and accessibility, the Hearing Aid Feature was evaluated in a clinical study that involved 118 participants across the US, all of whom had mild to moderate hearing loss. The users who used the self-fitting software achieved similar levels of hearing support as those who underwent professional fitting. The study also revealed comparable improvements in amplification levels and speech understanding, particularly in noisy environments, with no adverse events reported.
This FDA authorization follows the agency’s 2022 regulations, which allowed adults with mild to moderate hearing loss to purchase OTC hearing aids without a prescription.
Besides presenting as a discreet and versatile hearing aid, the AirPods Hearing Aid Feature allows real-time dynamic adjustments, enabling users to amplify conversations and environmental sounds while maintaining the high-quality audio experience that AirPods Pro is known for.
Apple’s hearing innovations will launch this fall, making the OTC Hearing Aid Feature available in over 100 countries, including the US, Germany and Japan.
As of 2024, the FDA has approved several OTC medical devices, including the Dexcom Stelo Continuous Glucose Monitor (CGM), the first OTC CGM system, which allows non-insulin users to monitor their glucose levels without a prescription.











Join or login to leave a comment
JOIN LOGIN