In late March, FDA Commissioner Scott Gottlieb announced the launch of the FDA’s Nutrition Innovation Strategy at the National Food Policy Conference in Washington D.C. Since then, the FDA has been working on developing strategies to promote public health through improved nutrition and supporting the development of healthier food options. On Tuesday, Gottlieb released a statement that discussed new steps that the FDA will take in order to advance health through their Nutrition Innovation Strategy.
In his statement, Gottlieb highlighted the connection between diet quality and health. The commissioner said that the FDA is working to ensure that consumers are aware of the health attributes associated with the products they are consuming.
“What we need is the policy framework that allows consumers to identify healthier options and the market forces to inspire the development of these opportunities at a cost that’s affordable,” said Gottlieb.
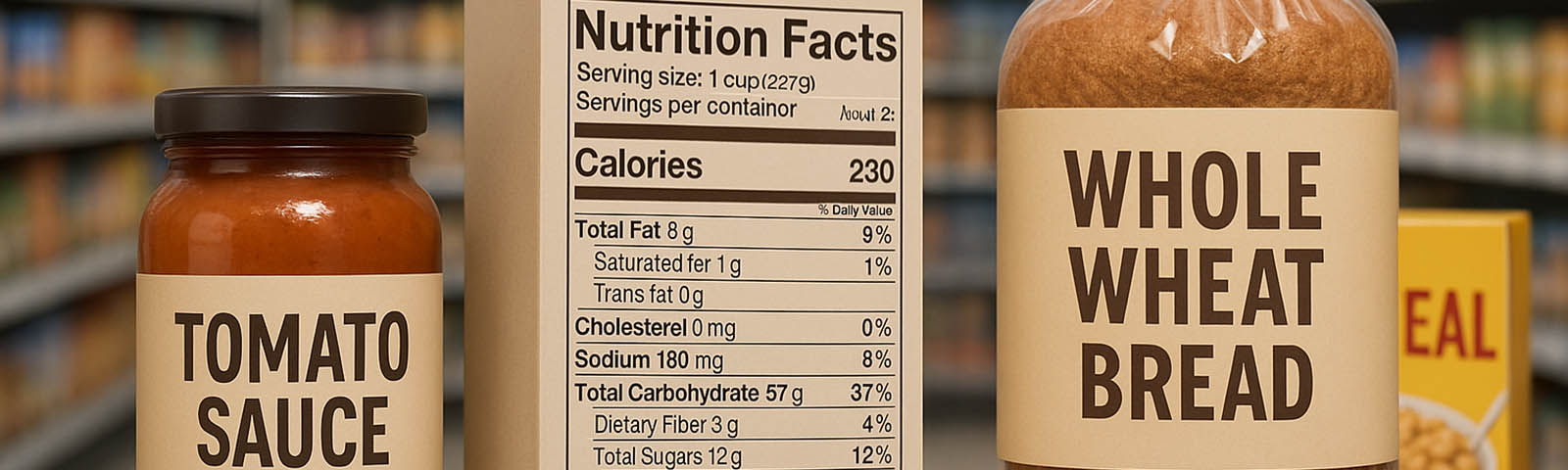
Gottlieb already realizes that consumers are actively seeking out healthier food options, however, he claims that consumers didn’t always have access to the information they need on a food product. The commissioner said that there is a need for science-based definitions for commonly used terms such as “healthy” or “natural” on food packaging. This issue is one of the major drivers behind the FDA’s multi-year Nutrition Innovation Strategy.
“This effort involves a series of new actions intended to modernize the FDA’s approach to nutrition, help reduce the burden of chronic disease that stems from poor nutrition, including obesity, diabetes, heart disease and a variety of cancers, and to remove barriers to industry innovation. Since the FDA regulates 80 percent of America’s food supply, we have a clear role to play in advancing policies that, in part, empower consumers with information when they’re making decisions about food,” he said.
Additionally, the FDA plans on providing incentives for food companies to produce healthier food options. In fact, the organization invited industry professionals, nutritionists and consumers to a public meeting on June 26th to discuss areas of their Nutrition Innovation Strategy. At the meeting, the organization highlighted their efforts to implement modernized food labels on product packaging and how these labels will help consumers identify healthy products. They also are looking into modernizing ingredient lists and standards of identity. In addition, the FDA announced that they are actively looking for incentives to provide to food companies that are compliant with their nutrition strategy.
According to Gottlieb, the FDA is thinking about producing a standard “healthy” logo in order to make healthy food options more visible to consumers. If this new logo is implemented, it could mean that food companies would have to certify the health qualities of their food products prior to labeling it as “healthy.” Regardless, the FDA plans on defining the term “healthy” in order to prevent false claims on packaging.
In regards to the ingredients list, the organization is looking for feedback on the idea of using simple terminology on ingredient lists instead of chemical names. This would allow consumers to easily identify healthy ingredients on food product labels.
“For example, manufacturers can make ingredient names more understandable by using terms like vitamin B6 instead of pyridoxine, or vitamin B12 instead of cyanocobalamin,” he said.
When it comes to standards of identity, which are standards that mandate the ingredients and manufacturing techniques of food, the organization is looking to modernize these standards in order to keep up with food innovations.
“To take one example, the standards of identity for certain cheeses don’t always permit the use of salt substitutes, which could be used to lower the sodium content of cheese. We’ve also been asked to modernize the standard of identity for yogurt to support the innovations occurring in this food category,” he said.
The FDA wants to know if consumers feel as if they are being misled when it comes to food alternatives that have the same names as their traditional counterparts. For example, products such as almond milk and cauliflower rice are not made from milk or rice, so this might be confusing for consumers.
The organization will be taking the options of consumers, food industry representatives and stakeholders into account before finalizing any new food mandates. Depending on the feedback they receive, the organization might start enforcing these new efforts to reduce false label claims. The FDA will also be implementing a full-scale educational campaign (through social media, websites and videos) for their new Nutrition Facts label in order to ensure that consumers are able to use it to its full potential.
“Leveraging nutrition as a way to advance public health remains one of my top priorities as Commissioner. All of these efforts represent the broad range of work the FDA is currently conducting to create a safe and healthier food supply for American families and to help consumers make more informed choices,” Gottlieb said. “Continued input on this initiative will be key as we use the tools of diet and nutrition to advance our public health goals. With these science-based efforts, we’re committed to promoting the availability of healthy dietary choices for all Americans.”










Join or login to leave a comment
JOIN LOGIN