The first active osseointegrated steady-state implant, Osia 2 System by Cochlear, has been approved by the US Food and Drug Administration (FDA). According to Cochlear, this is a new category of bone conducted hearing solution that “uses digital piezoelectric stimulation to bypass damaged areas of the natural hearing system to send sound vibrations directly to the inner ear (cochlea).”
The Osia System is developed by Cochlear, a leader in implantable hearing solutions. The system is designed to treat hearing impaired individuals that have conditions associated with chronic otitis media, otosclerosis, conductive hearing loss, mixed hearing loss and single-sided sensorineural deafness.
“At Cochlear we have been working to develop this technology for quite some time,” said Mats Dotevall, director of design and development and director of clinical affairs at Cochlear Acoustics.
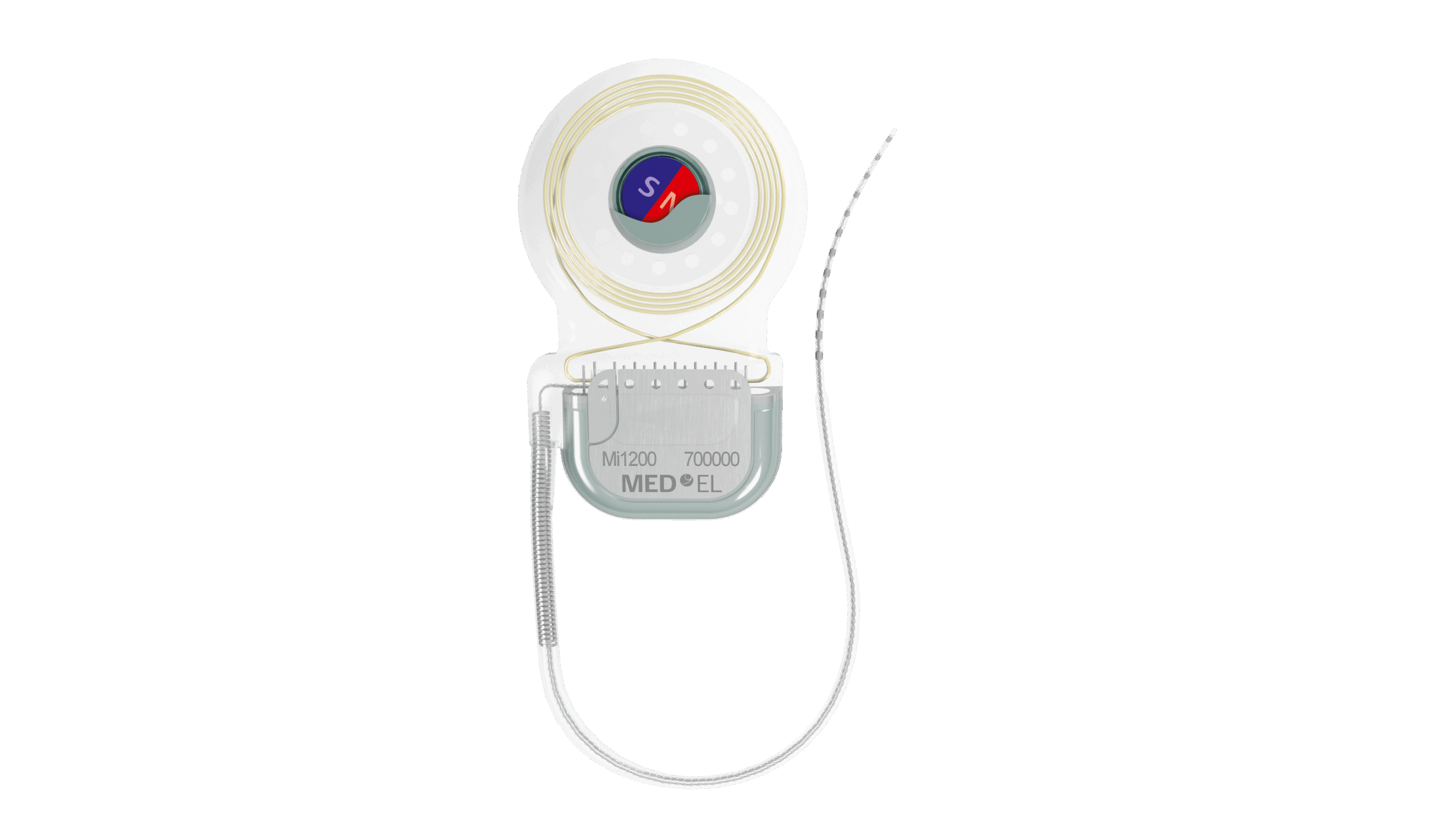
The device is made of two parts, one being the sound processor and the other being the implant. The implant is placed behind the ear and under the skin, while the processor sits just off of the ear. They are held by a magnetic connection. The microphones capture sound, the processor converts the sound to a digital signal, and the signal is sent through the skin through a digital link.
The signal then moves down the implant to the Piezo Power transducer. The transducer that’s placed behind the ear generates mechanical vibrations by using the piezoelectric material. The titanium implant, which is in the bone, transmits vibrations to the inner ear through the skull, where the inner ear converts the vibrations into electrical impulses. Finally, the impulses are sent to the brain to be interpreted as sound.

The system is powered by a wireless digital link that amplifies the frequencies of sound to a higher level.
“We aimed to leverage Cochlear’s long history of innovation in both the cochlear implant and bone conduction implant spaces to create something entirely new. Rather than implanting a conventional electromagnetic transducer, we chose to work with piezoelectric material because of its unrivalled suitability for this type of implanted application. Lifetime testing shows Piezo Power technology provides powerful and consistent performance over time,” said Dotevall.
The Osia 2 system will be available in select clinics across the US. Health Canada’s approval for the Osia 2 System is expected to arrive in early 2020.










Join or login to leave a comment
JOIN LOGIN