Chronic kidney disease (CKD), an often silent yet serious medical condition, affects more than 10 percent of the global population. Alarmingly, the cardiac ramifications of CKD are deep and manifold, and with cardiovascular disease (CVD) standing as the leading cause of death worldwide, it has especially important implications among CKD patients.
“The prevalence of CVD can be upwards of 30 times higher in those with CKD and also we can see a higher chance of 3 vessel coronary artery disease,” says Dr. Ajay Srivastava, MD, FASN, Sr. Vice President of the Medical Department and Nephrology therapeutic lead at Medpace.
These statistics for CKD patients are alarming as Dr. Srivastava highlights the disheartening facts related to mortality including that a CKD patient is more likely to die from CVD before ever having progressed to requiring dialysis, a life-saving therapy for those with end-stage kidney disease (ESKD), emphasizing the urgent need for increased research in this area.
Yet, despite the overwhelming evidence linking CKD with this heightened cardiovascular risk, CKD patients are often underrepresented or outright excluded from cardiovascular clinical trials.
Historically, Dr. Srivastava notes, CKD has often been considered a potential exclusion criterion for clinical trials due to apprehensions regarding the possibility of altered drug pharmacokinetics and the perceived risk of a worsened prognosis in patients with CKD. This exclusion not only narrows the understanding of CVD in the presence of CKD but can also hinder the potential for therapeutic advances that would have wide-ranging applications across a spectrum of potential patients.
Dr. Srivastava recently spoke on a webinar where he shared insights on the imperative need to bridge this research gap and include more CKD patients in cardiovascular clinical trials. He was joined by his colleagues at Medpace, Dr. Nicholas Alp, MD, PhD, FACC, Sr. Vice President of the Medical Department and Cardiology therapeutic lead, and Heather Lohr, Sr. Director of Clinical Trial Management. Watch the free webinar to hear their expert insights.
Read on to learn how the inclusion of CKD patients in cardiovascular clinical trials can pave the way for more comprehensive treatments that cater to a wider patient population, and ultimately, not only save more lives, but improve the quality of those lives.
An Urgent Focus on Including CKD Patients in Drug Development
CKD is a growing public health concern, surging in both frequency and prevalence. As CKD progresses, Dr. Srivastava emphasized that patients experience an increased risk of various adverse outcomes, including and especially cardiovascular morbidity and mortality. CVD is the leading cause of death in the world and has been so in the United States for well over a century.
Our understanding of the relationship between CKD and CVD has evolved over time, which has elucidated the fact that traditional risk factors for CVD in the general populace don’t fully encapsulate the heightened cardiovascular mortality seen in CKD and end-stage kidney disease (ESKD) patients. Other than age, the other traditional risk factors such as dyslipidemia, smoking, diabetic control, hypertension, obesity and metabolic syndrome are modifiable while CKD introduces additional risk factors. Conditions often seen in CKD patients — albuminuria, malnutrition, anemia, mineral metabolic disorders and others — are also potent CVD risk factors (Figure 1).
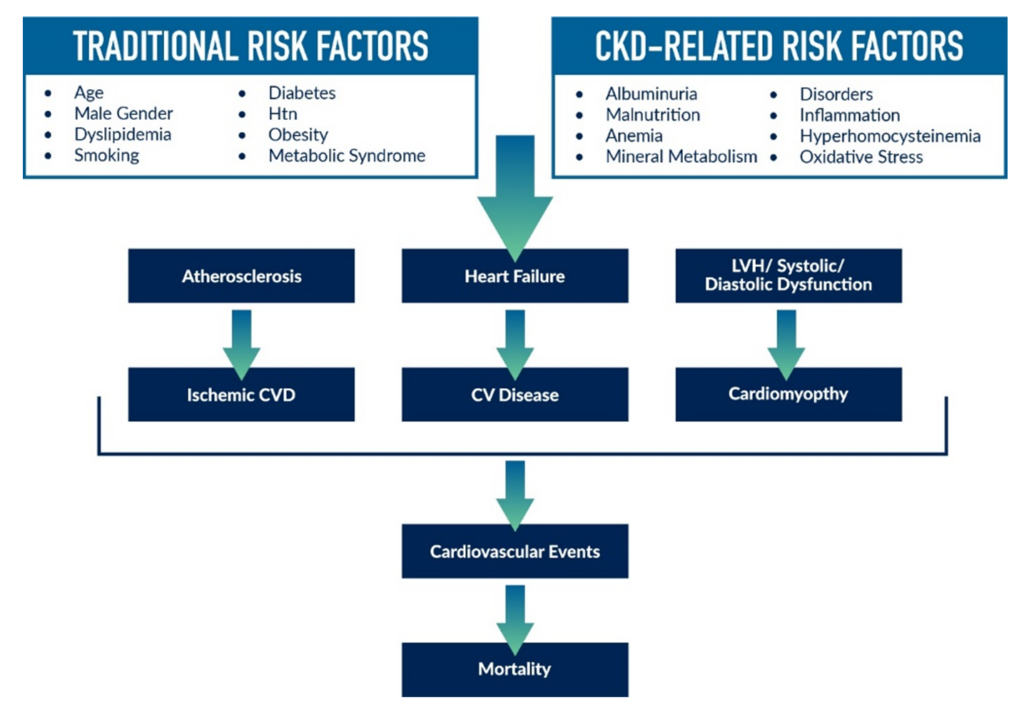 Figure 1. Traditional cardiovascular risk factors and CKD-related cardiovascular risk factors. Adapted from Liu et al. Eur Rev Med & Pharmacol Sci. 2014. 18: 2918-2926.
Figure 1. Traditional cardiovascular risk factors and CKD-related cardiovascular risk factors. Adapted from Liu et al. Eur Rev Med & Pharmacol Sci. 2014. 18: 2918-2926.
This revelation underscores the urgency for targeted research and clinical trials.
As such, Dr. Alp notes that “Today, we must ensure that patients with progressive CKD are a part of our clinical research.”
Both Dr. Srivastava and Dr. Alp stress the importance of comprehensive and collaborative care, recognizing that a better understanding of the management of CVD in the CKD patient is of paramount importance, especially given the close interdependence of the heart and kidneys and the resulting ramifications of this relationship.
The Synergy between Heart and Kidney Health
“The heart and the kidneys sink and swim together,” Dr. Alp says, referring to the fact that the heart and kidneys are closely linked through a complex network of endocrine interactions.
Contrary to the layperson’s belief of the heart and kidneys being a mere pump and filtering system respectively, Dr. Alp stressed that “it’s a finely balanced and tuned endocrine system, aiming to preserve fluid balance and chemical homeostasis,” with both organs directly impacted by common, chronic conditions such as type 2 diabetes, hypertension and atherosclerosis.
It becomes especially concerning when one of these organs is damaged, in which the other is invariably affected. This refers to cardiorenal syndrome (CRS), a complex interaction between the heart and kidneys, where an acute or long-term dysfunction in one organ can lead to acute or long-term dysfunction in the other organ.
There are several classifications or subtypes of CRS, which are generally divided into five types based on the primary organ dysfunction (heart or kidney) and the acuity (acute or chronic) of the syndrome.
Dr. Alp delved into the intricacies of type 2 and type 4 CRS (Figure 2), both of which refer to chronic conditions. The mechanisms driving these syndromes range from hemodynamic factors like tissue perfusion to nonhemodynamic drivers like inflammation and oxidative stress. “Once this cycle starts, it’s a spiral. It should be a focal point of future drug development and clinical research,” says Dr. Alp.
Type 2 CRS is chronic cardiorenal syndrome. This refers to chronic heart disease (e.g., chronic heart failure) leading to CKD. Over time, chronic heart dysfunction results in progressive and permanent kidney injury.
Type 4 CRS is chronic renocardiac syndrome. Here, CKD (due to factors like longstanding hypertension or diabetes) contributes to decreased cardiac function with an increased risk for cardiovascular events.
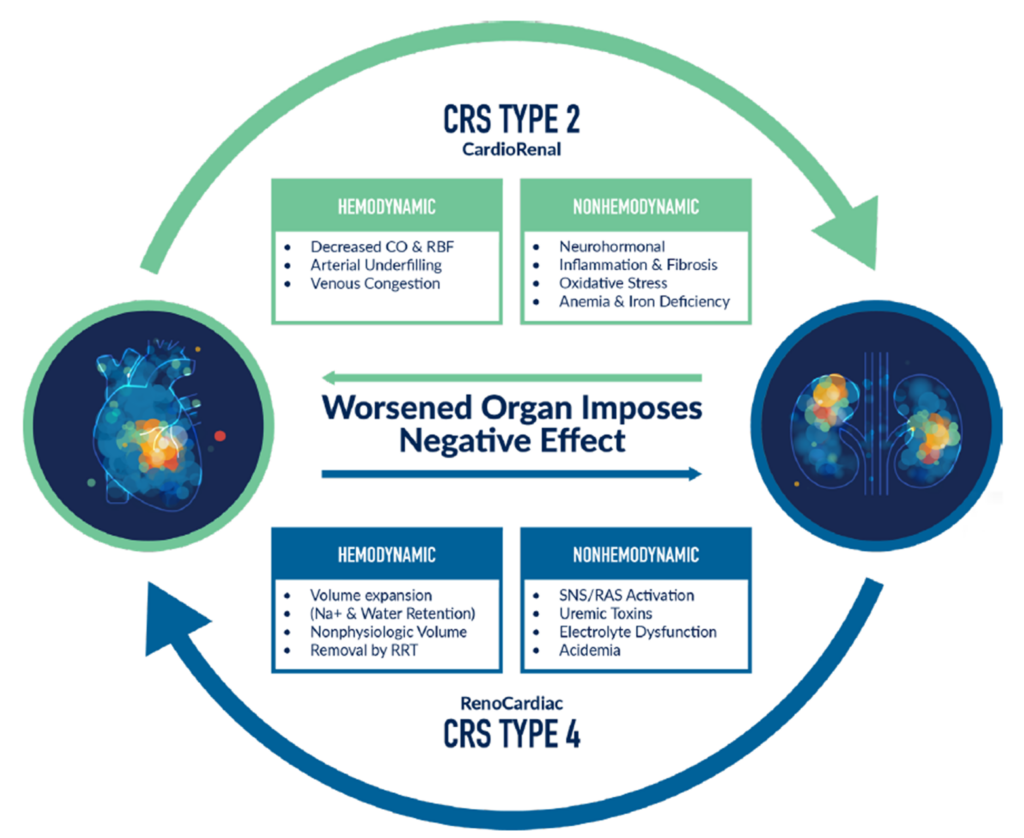
Figure 2. The interconnection between type 2 and type 4 cardiorenal syndrome (CRS). CO = cardiac output; RBF = renal blood flow; Na+ = sodium; RRT = renal replacement therapy; SNS = sympathetic nervous system; RAS = renin angiotensin system. Adapted from Srivastava A, et al. “Cardiorenal Syndrome Type 2” Critical Care Nephrology, 3/E, Edited by C. Ronco, R. Bellomo, J.A. Kellum & Z. Ricci. 2018. 690-701; and from Jentzer J, et al. JACC. 2020. 76(9): 1084-1101.
Given the complex relationship between heart and kidney function, patients with CRS often require close monitoring and collaboration between cardiologists and nephrologists with management epitomizing a multi-disciplinary approach.
Reflecting on his years of practice, Dr. Alp recalled, “I noticed in my early career that cardiologists and nephrologists worked largely independently… there wasn’t a lot of integration or cross-talk between them.” But there’s hope, as the emergence of cardiorenal specialists seeks to bridge this divide. According to Dr. Alp, these specialists are essential for progress in clinical practice and drug therapeutics.
Understanding the Cardiorenal Concept: A Clinical Trial Case Study
Drug development has of late been embracing the cardiorenal concept with the advent of new drugs and the rediscovery of existing ones that may benefit those with CKD and its associated comorbid diseases.
Dr. Srivastava shed light on a recent clinical trial that exemplified the importance of this concept.
Baxdrostat, a novel aldosterone synthase inhibitor (ASI), has emerged as an important and potentially important addition to the arsenal of medications in the management of treatment-resistant hypertension. With a notable population struggling to control blood pressure despite maximal treatment regimens, this compound has drawn substantial interest amongst the healthcare community.
This sentiment is held close to the hearts of Dr. Srivastava, Dr. Alp and their colleagues, who have directly contributed to advancing our understanding of baxdrostat through their involvement in a multicenter, randomized Phase II clinical trial on treatment-resistant hypertension. This Phase II trial was designed to compare the effects of baxdrostat or placebo on patients with treatment-resistant hypertension who were receiving stable doses of at least three antihypertensive agents, including a diuretic.
One of the key outcomes of the study was a substantial decrease in blood pressure among these patients treated with baxdrostat. The promising results point towards the effectiveness of baxdrostat as an adjunct to antihypertensive therapy in the treatment-resistant population.
Historically, many hypertension trials have excluded patients with significant kidney disease due to concerns regarding drug safety and effectiveness in this population. Importantly, this study permitted the enrollment of patients with an estimated glomerular filtration rate (eGFR) down to 45 mL/min/1.73 m2, corresponding to stage 3 CKD. This inclusion is particularly significant, as uncontrolled or treatment-resistant hypertension is more prevalent in CKD populations, often related to issues with volume overload.
The decision to include patients with stage 3 CKD in the trial is noteworthy for a few reasons:
- Treatment options for CKD patients: The inclusion of these patients may pave the way for broader use of ASIs like baxdrostat in CKD populations, where treatment-resistant hypertension is more likely and has important negative downstream effects.
- Safety profile: As ASIs block aldosterone production, they could indirectly affect potassium homeostasis. The study was especially concerned with potential hyperkalemia (elevated potassium levels), a condition that could have serious and acute cardiac effects. The fact that no patients had to discontinue the trial due to potassium elevation suggests that baxdrostat may have a favorable safety profile, including those patients with reduced renal function, who are at higher risk of hyperkalemia.
- Potential for further research: The positive outcomes of this Phase II trial, in conjunction with the inclusion of patients with significant CKD, may encourage further research. These future studies could solidify baxdrostat’s role as a vital tool in managing treatment-resistant hypertension, particularly in populations with concurrent kidney disease.
The study involving baxdrostat, as described by Dr. Srivastava, Dr. Alp and colleagues, showcases the potential of this ASI as a crucial new tool in the fight against treatment-resistant hypertension as well as other nuanced uses related to the aldosterone reducing effects of this drug.
Given the increasing prevalence of both hypertension and CKD globally, this work contributes significantly to the medical community’s quest for better, safer and more inclusive treatment options.
Medpace’s One-Team Approach: Integrating Cardiorenal Teams
Dr. Srivastava shed light on Medpace’s one-team approach to cardiorenal integration in clinical trials and how it stands to help revolutionize the medical monitoring landscape.
“We’re looking at it from both an operational perspective, as well as a medical monitoring perspective where we’re integrating cardiovascular and renal teams,” says Dr. Srivastava.
Central to this integration, Dr. Srivastava notes, is the collaboration with Medpace Reference Laboratories (MRL), a central lab that has been fundamental in providing world-class expertise, seamless processes and optimized efficiencies while also supporting innovation. Dr. Srivastava discusses the significant advancements made toward 24-hour urine collections for endpoint protection, where lab parameters can quickly identify potentially inappropriate 24-hour collections for medical monitor evaluation, thus ensuring the integrity of study results.
Visual analytics are also crucial as part of aggregate safety reviews, particularly for studies that heavily rely on labwork as are commonly seen in integrated studies. Data visualization dramatically helps in the analysis and assessment with regard to the identification of potential safety signals and ensures that teams can effectively communicate any concerns (Figure 3).
Figure 3. Safety data review with visual analytics is vital for cardiorenal teams.
Delving deeper into medical monitoring perspectives, Dr. Srivastava elaborated on these crucial elements that involve collaboration:
- The priority in forged team collaboration, efficient communication and established project plans
- Emphasizing the importance of integrated functional areas
- Ensuring accurate and expeditious assignment and rapid review of real-time lab alerts
- Maintaining a global presence that is consistently effective including weekends or holidays
Every study benefits from specialized expertise. As Dr. Srivastava says, “Collaboration is the cornerstone of success. It’s the integration and successful collaboration of medical and operational expertise and perspectives that determine the success of critically important trials in areas such as team training, regulatory compliance and patient recruitment.”
Managing Cardiovascular Clinical Trials with CKD Patients
Cardiovascular clinical trials involving CKD patients are notable for unique challenges. Mrs. Lohr emphasizes the importance of early and consistent engagement with investigators, patient-centric study designs and effective communication for successful trial execution.
Early Engagement with Investigators Is Critical
CKD and CVD patients frequently present with a myriad of comorbidities which makes them a particularly complex population to manage. Therefore, it is necessary to engage medical and operational teams with investigators at an early stage in protocol development. This ensures that the study design can be implemented successfully in this population.
“The investigators will be able to provide critical insight and confirm whether or not the study design is feasible and what limitations may need to be overcome,” Mrs. Lohr says.
These engagements allow study teams to understand how patients present at the site, their current management protocols, the standard of care they are receiving and the infrastructure required to run the study so that they in turn can develop a study design that is operational
Adopting a Patient-Centric Approach
It is important to adopt a patient-centric approach to successfully enroll a study with CKD and CVD patients.
“We found that it’s important to provide practical, creative and flexible solutions to meet the needs of each patient,” Mrs. Lohr says. “This may include allowing remote consent and remote visits, or even direct patient shipping of the investigational product (IP).”
Mrs. Lohr shares practical ways to ease patient participation, such as specialized courier services for urine samples, minimizing discomfort in assessments and considering the timing of study procedures to align with patients’ treatments.
“For example, allowing certain assessments to occur when the patient is receiving their dialysis treatment can shorten the length of their stay in the clinic,” Mrs. Lohr suggests.
This approach includes planning for a positive patient experience to reduce disruptions for patients and caregivers, limiting unnecessary procedures and allowing for certain medications to continue if not contraindicated.
Despite starting with known patients, the patient pool for cardiorenal trials often shrinks due to various reasons, such as competing trials or hesitancy to change current therapy (Figure 4).
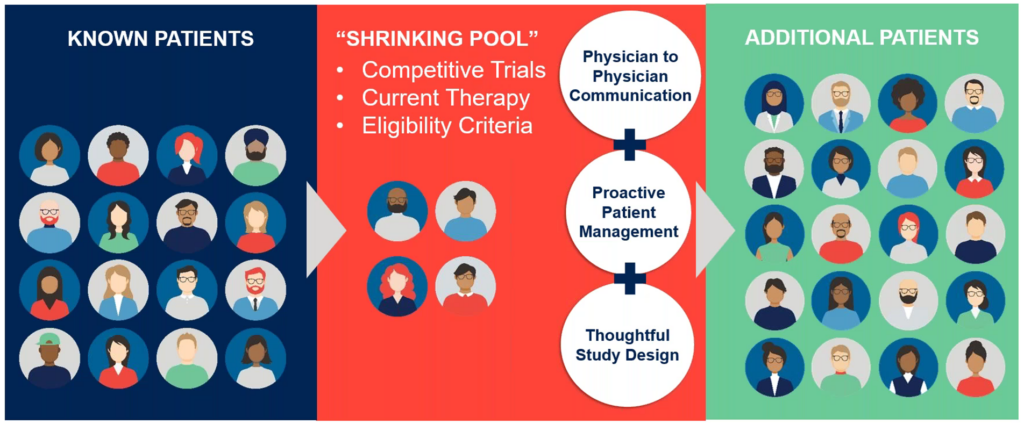
Figure 4. To maintain participant numbers in cardiorenal trials, additional recruitment strategies should be implemented as the initial patient pool frequently diminishes for various reasons.
According to Mrs. Lohr, this “shrinking pool” can be mitigated by fostering strong communication between investigators and medical monitors, while maintaining active engagement from the operational team.
“Proper communication and proactive patient management ensure not only the right patients are enrolled but that they are able to continue in the study,” says Mrs. Lohr.
Importance of Cross-Functional Collaboration
Cross-functional collaboration is also essential when setting up a study. This involves integrating the expertise of the medical monitors, operational leads, central lab, data management and safety teams.
Mrs. Lohr touches on the critical aspects of setting up study systems. For instance, she underscores that it is vital for relevant lab and safety alerts to be received by investigators, medical monitors, clinical leads and clinical research associates (CRAs), emphasizing the essential role of effective communication and proactive patient management.
Mrs. Lohr provided a real-life example from a dialysis study that involved complex dose modifications based on lab parameters. “It required close collaboration between the sites, medical monitors and clinical operations to ensure the dose adjustments were performed correctly, so the patients could remain in the study,” she said.
As the landscape of clinical research evolves, these operational perspectives provide a roadmap for fostering patient trust and ensuring data integrity.
In the intricate balance between the heart and kidneys, understanding their complex and intricate relationship in order to develop treatments that address their mutual challenges is paramount. The expertise and insights offered by the team at Medpace provide a beacon of hope in this endeavor.




![Xtalks Hero Image - RDD Webinar 2024 [Recovered]](https://xtalks.com/wp-content/uploads/2024/01/Xtalks-Hero-Image-RDD-Webinar-2024.jpg)
Join or login to leave a comment
JOIN LOGIN