In a move that is reflective of a significant milestone in the field of digital cytology, Hologic has launched the Genius Digital Diagnostics System. This system can help revolutionize how healthcare professionals approach cytology by offering enhanced capabilities and efficiency in diagnostic processes. It combines deep-learning-based artificial intelligence (AI) with advanced volumetric imaging technology to help in the diagnosis of pre-cancerous lesions and cervical cancer cells.
Recently, Hologic has received approval for the Genius Digital Diagnostics System from the US Food and Drug Administration (FDA). It is the first and only FDA-approved digital cytology solution in the market.
“Hologic is a leading innovator in women’s health with a commitment to advancing cervical and breast cancer screening technologies, from the first liquid-based cytology test to the first 3D mammography system and now the first FDA-cleared digital cytology platform,” said Jennifer Schneiders, PhD, president, Diagnostic Solutions at Hologic in the company’s press release.
XTALKS WEBINAR: Infectious Disease Diagnostics for Influenza, RSV and SARS-CoV-2
Live and On-Demand: Tuesday, March 5, 2024, at 12pm EST (5pm GMT/UK)
Register for this free webinar to learn how to effectively take an infectious disease diagnostic assay from initial design stages through regulatory approvals that support market access.
In its most recent update, the American Cancer Society estimated that 13,820 cases of invasive cervical cancer will be diagnosed in the US in 2024, and approximately 4,360 women will die from the disease.
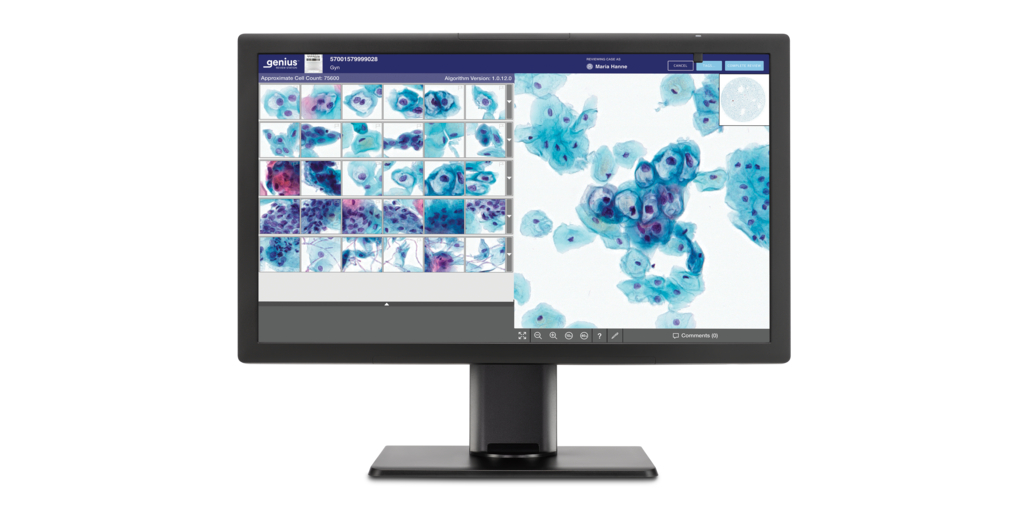
Detecting and identifying cervical cancer in the earliest stages is critical to effective prevention and treatment. Generally, to screen for cervical cancer, a Pap test is performed for which a sample is usually collected at the OB-GYN office, and then the cervical cells are sent to a lab where they are transferred to a glass slide. To date, these slides are then reviewed under a microscope. However, with the Genius Digital Diagnostics System, the glass slides are digitally imaged and an AI algorithm is applied to identify the cells that cytologists and pathologists should review.
Cervical cancer can be prevented and, if caught early, can be highly treatable. Co-testing, (combining a Pap test with a human papillomavirus [HPV] test), is the most sensitive testing option for cervical cancer screening compared to either test used alone. Hologic pioneered the first FDA-approved liquid-based cytology test, the ThinPrep Pap test, and the first FDA-approved mRNA-based HPV test, the Aptima HPV Assay. Healthcare professionals have the choice to perform a co-test with ThinPrep and Aptima.
Related: Aptima CMV Quant Assay Gets FDA Nod for Cytomegalovirus Detection in Transplant Patients
Improving Efficiency in Diagnosis with the Genius Digital Diagnostics System
The Genius system demonstrated an overall improvement in sensitivity without any reduction in specificity. In fact, compared to microscopic review, a 28 percent reduction in false negatives of high-grade squamous intraepithelial and more severe lesions was observed.
The system is expected to assist laboratories in keeping their healthcare professionals informed and guide them in making timely and effective treatment decisions for their patients. Cytologists and pathologists can securely review cases in a remote setting, and thus patients have the benefit of getting treatment options and suggestions from geographically dispersed experts.
“Our technologies have had a tremendous impact on decreasing cancer rates in women, and we are incredibly excited by the promise of Genius Digital Diagnostics. The system delivers more actionable and accurate insights for laboratories and healthcare professionals to enhance patient care,” said Schneiders.
The Genius Digital Diagnostics System comprises the Genius Digital Imager for image acquisition, the Genius Cervical AI algorithm for image analysis, the Genius Image Management Server for image storage and the Genius Review Station for local or remote case review.
The introduction of this innovative digital cytology system is anticipated to have a positive impact on patient care as swift and accurate diagnoses enable timely interventions and treatment strategies, contributing to improved overall healthcare outcomes.
In the field of digital diagnostics and cytology systems, Roche’s Digital Pathology solution is a direct competitor. Other companies, such as Abbott Laboratories and Becton, Dickinson and Company (BD), may also pose significant competition as they advance their own digital diagnostics solutions. The choice between these systems often depends on specific features, technological nuances and the preferences of healthcare professionals and organizations.











Join or login to leave a comment
JOIN LOGIN