Update (August 30, 2018): AI diagnostics company IDx has now published results of a trial assessing the safety and efficacy of their IDx-DR diabetic retinopathy detection system in the journal Nature Digital Medicine. The pivotal trial involved 900 patients with diabetes and found that the system showed 87 percent sensitivity and 90 percent specificity at diagnosing diabetic retinopathy.
Originally published on April 13, 2018:
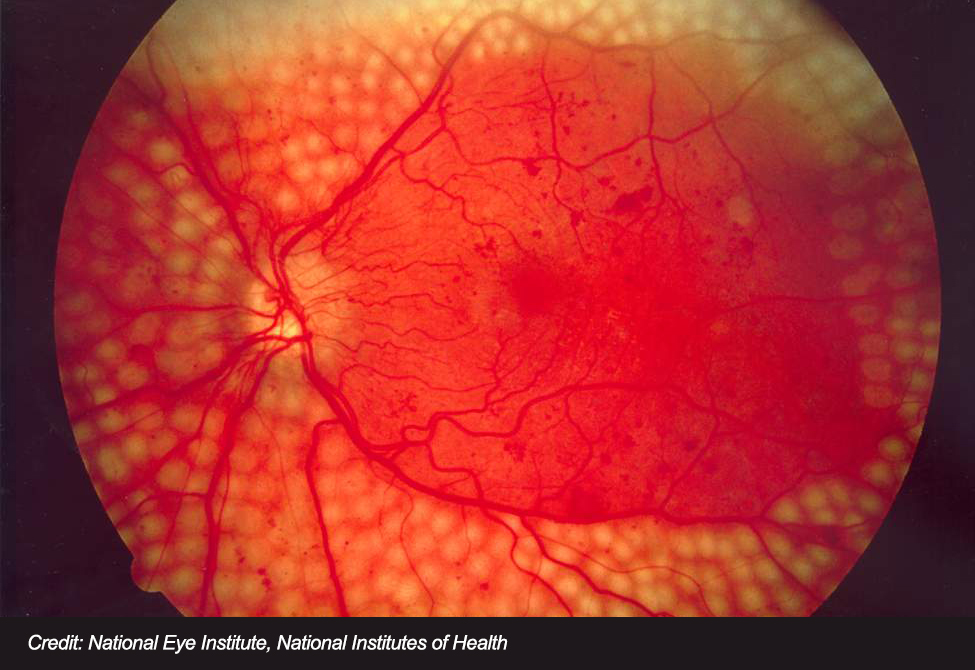
The first artificial intelligence (AI) based diagnostic system – the IDx-DR – has been cleared by the US Food and Drug Administration (FDA) to detect diabetic retinopathy during a patient’s routine visit to their physician’s office. With over 30 million individuals in the US having been diagnosed with diabetes, the risk of developing diabetic retinopathy is significant for nearly 10 percent of the population. According to the CDC, around 24,000 diabetics will lose their sight as a result of diabetic retinopathy, which can be prevented if diagnosed early.
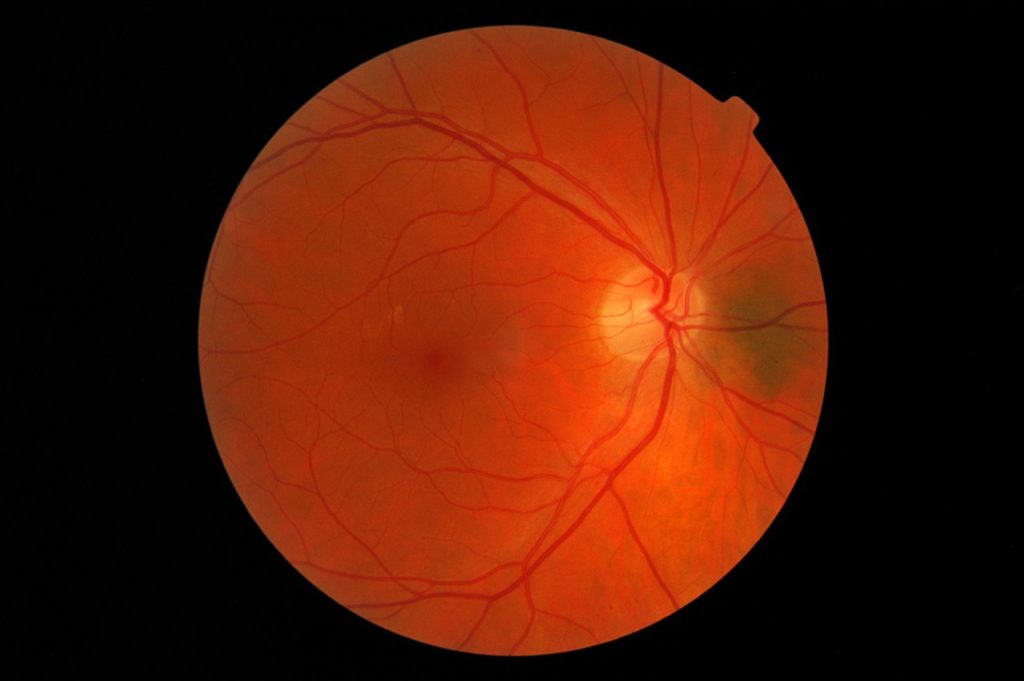
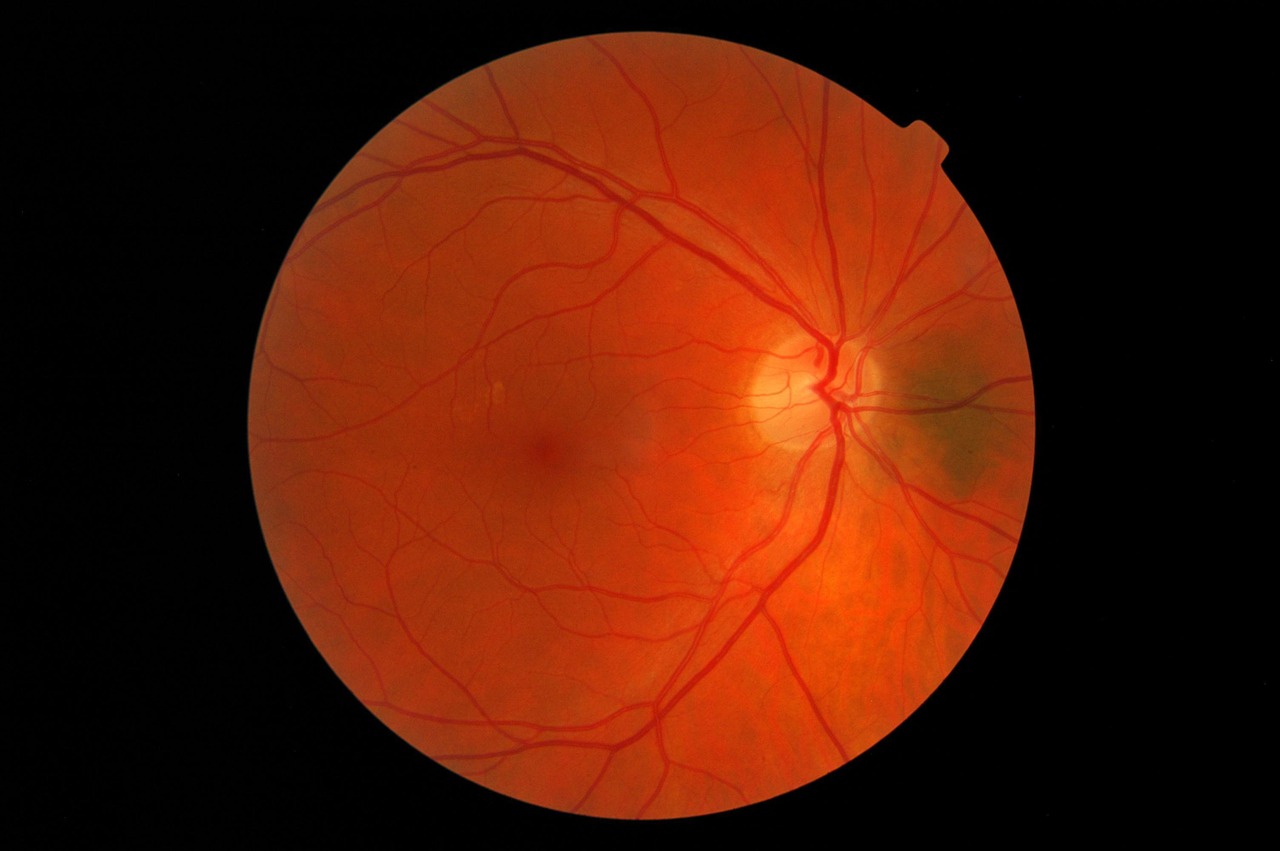
Like most complications of diabetes, diabetic retinopathy is caused by uncontrolled blood sugar levels that damage retinal blood vessels. This, in turn, damages the light-sensitive cells in the eye leading to vision problems and eventual blindness.
“The FDA’s authorization to market IDx-DR is a historic moment that has the potential to launch a transformation in the way US healthcare is delivered,” said Dr. Michael Abràmoff, founder and president of IDx. “Autonomous AI systems have massive potential to improve healthcare productivity, lower healthcare costs, and improve accessibility and quality. As the first of its kind to be authorized for commercialization, IDx-DR provides a roadmap for the safe and responsible use of AI in medicine.”
IDx-DR software analyzes a patient’s retinal images using an AI algorithm and provides a diagnosis in just a few minutes. After evaluating the images for signs of diabetic retinopathy, the software returns one of two results: “more than mild diabetic retinopathy detected: refer to an eye care professional,” or, “negative for more than mild diabetic retinopathy; rescreen in 12 months.”
RELATED: Diabetic Retinopathy Could Soon be Diagnosed by AI System
While clinicians have long used computer programs to help with diagnosis, the IDx-DR is the first tool that doesn’t require a doctor’s input. This means that the diabetic retinopathy AI-device can be used in a primary care setting where physicians may not have ophthalmic expertise.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy since about 50 percent of them do not see their eye doctor on a yearly basis,” said Dr. Malvina Eydelman, director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA’s Center for Devices and Radiological Health. “Today’s decision permits the marketing of a novel artificial intelligence technology that can be used in a primary care doctor’s office. The FDA will continue to facilitate the availability of safe and effective digital health devices that may improve patient access to needed health care.”
The company demonstrated the clinical effectiveness of the IDx-DR by conducting a multi-center clinical trial involving 900 patients and 10 primary care facilities. The software successfully detected patients with more than mild diabetic retinopathy 87.4 percent of the time, while patients without the eye disease were correctly identified 89.5 percent of the time. The FDA granted the IDx-DR Breakthrough Device designation, which expedited the review of the diagnostic.
“I am excited that IDx-DR is now cleared for use; there is a definite need for a more affordable and accessible option for the early detection of diabetic retinopathy,” said Dr. Michele Birch, a primary care physician at CMC-Elizabeth Family Medicine and the University of North Carolina School of Medicine. “Making sure that my patients with diabetes have annual eye exams has always been a challenge, but IDx-DR can make it easier by allowing me to conduct an exam right in my office.”



Join or login to leave a comment
JOIN LOGIN