Olympus Corporation, a prominent manufacturer specializing in optics and reprography products with headquarters in Japan, has recently revealed compelling evidence demonstrating the effectiveness of the minimally invasive iTind procedure. The published study results revealed that this revolutionary procedure delivers long-lasting relief extending beyond four years for individuals suffering from symptoms associated with benign prostatic hyperplasia (BPH), commonly referred to as an enlarged prostate.
“This evidence of clinical durability is important to physicians considering surgical options for BPH symptom treatment,” said Daniele Amparore, MD, Division of Urology, San Luigi Hospital, Orbassano, Italy, and co-lead author of the study, in the company’s news release. “We now have published study data showing iTind device treatment is a reliable minimally invasive surgical option with minimal safety concerns for extended time periods.”
In 2020, the iTind (Temporarily Implanted Nitinol Device) was granted de Novo Authorization by the US Food and Drug Administration (FDA) for the non-surgical treatment of BPH.
BPH is a common health condition that often arises as men age. It impacts a significant number of men, with around 50 percent of individuals aged 51 to 60 experiencing it, and prevalence increasing up to 90 percent for men over 80. Symptoms frequently associated with BPH include frequent urination, a sense of urgency, a weak urinary stream and excessive nocturnal urination. Over time, these symptoms can severely affect men’s quality of life and that of their families.
How Does iTind Work?
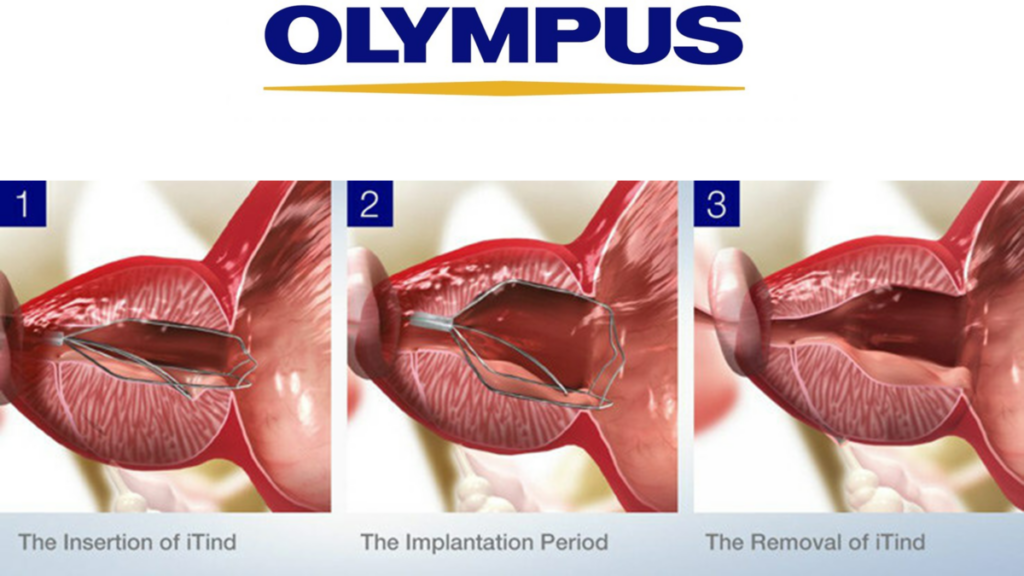
The iTind procedure is an innovative and minimally invasive treatment intended for individuals aged 50 and above experiencing lower urinary tract symptoms due to BPH. The procedure aims to reshape the prostate, creating a broader opening for urine to flow freely, without needing to burn or cut out prostate tissue, and without leaving a permanent implant.
The iTind device is temporarily implanted to reshape the prostatic urethra during the procedure. This can be performed in an outpatient setting or a physician’s office. The device stays in place for five to seven days while the patient is at home. Upon removal of the device, patients usually experience rapid and effective symptom relief. The procedure offers a non-surgical approach with significant benefits in symptom alleviation and improved urinary function.
XTALKS WEBINAR: How the Recent FDA & EMA Regulations are Helping Advance and Enhance Electronic Clinical Outcome Assessments (eCOA/ePRO)
Live and On-Demand: Wednesday, July 26, 2023, at 11am EDT (4pm BST/UK)
Register for this free webinar to learn how new guidances from European and US regulators have the potential to enhance and accelerate the use of eCOA/ePRO in clinical trials.
The Benefits of iTind
The iTind procedure offers numerous advantages, such as immediate symptom relief and eliminating the need for postoperative catheterization. Unlike permanent implants, the iTind device can be safely removed after five to seven days. The procedure also potentially preserves sexual function.
Strong clinical evidence supports the benefits of the iTind procedure. In a prospective, single-arm, multicenter study, the iTind device treatment demonstrated significant symptom improvement, with a notable 45.3 percent reduction in the International Prostate Symptom Score (IPSS) and a 45.1 percent reduction in the IPSS-Quality of Life (IPSS-QoL) from baseline to 79 months after the procedure (both p < 0.0001).
Importantly, no late postoperative complications were reported beyond the 36-month follow-up period, and no patients required additional medication. The surgical re-treatment rate after 36 months was 4 percent, and the cumulative re-treatment rate from baseline to 79 months reached 11.1 percent.
The Risks of the iTind Procedure
While undergoing the iTind procedure, patients may experience mild to moderate side effects, including urinary urgency, pelvic discomfort, dysuria (difficult or painful urination) or hematuria (blood in urine). Rarely, there may be a risk of urinary tract infection or acute urinary retention. Most side effects occur during the five-to-seven-day treatment period and resolve shortly after device removal.
The iTind procedure may not be the optimal choice for all patients. Comprehensive consultations with healthcare professionals are crucial to assess individual circumstances and determine if the iTind procedure is the most suitable option. The guidance of medical professionals is essential for informed decision-making about the appropriateness of the iTind procedure.
Alternative Treatments for Benign Prostatic Hyperplasia (Enlarged Prostate)
Several treatments have received clearance from the FDA or are undergoing advanced clinical development for BPH management. One such treatment is the UroLift System, developed by Teleflex Incorporated, a leading American provider of specialized medical devices. This system gained FDA clearance for BPH treatment in 2020.
The UroLift System is a proven, minimally invasive technology designed to treat lower urinary tract symptoms caused by BPH. UroLift implants, delivered through a transurethral outpatient procedure, relieve prostate obstruction and directly open the urethra without cutting, heating or removing prostate tissue. Clinical data from a pivotal randomized controlled study involving 206 patients demonstrated that the UroLift implants led to rapid and long-lasting symptom improvements without compromising sexual function.
Additionally, Aquablation therapy, developed by the surgical robotics company PROCEPT BioRobotics, received FDA clearance in late 2017. Aquablation therapy stands out due to its unique heat-free waterjet, controlled by robotic technology, which precisely removes prostate tissue. By combining a cystoscope camera with ultrasound imaging, the surgeon has real-time visibility of the entire prostate during the procedure. Thus, Aquablation therapy offers high precision, consistency and predictability, providing long-term relief regardless of prostate size. Importantly, this therapy has a notably low rate of irreversible complications, such as incontinence, ejaculatory dysfunction and erectile dysfunction. Clinical results have demonstrated Aquablation therapy’s ability to provide long-term relief for up to three years with minimal risk of irreversible complications.











Join or login to leave a comment
JOIN LOGIN