Due to an undisclosed problem at a Pfizer-owned manufacturing facility, Canadian patients with severe allergies are facing a shortage of Mylan’s EpiPen. Health Canada received word from Pfizer about the supply shortage this month, and while the manufacturer plans to distribute some of its limited inventory of the EpiPen next month, this manufacturing issue might not be resolved until March.
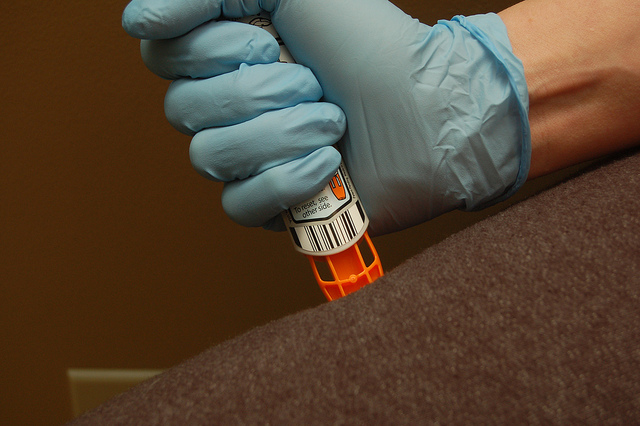
The EpiPen is used for the emergency treatment of anaphylaxis in patients with severe allergies. The combination device consists of an auto-injector syringe prefilled with epinephrine.
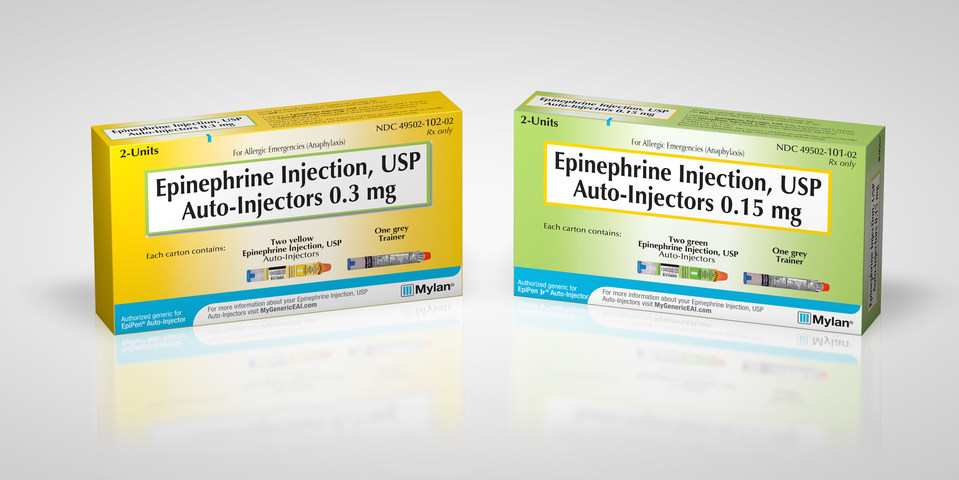
The shortage only affects 0.3mg EpiPens, and is not expected to affect the EpiPen Jr. – the pediatric version of the auto-injector. Since there are no EpiPen alternatives available in Canada, patients unable to fill their prescription may have to go without the lifesaving epinephrine injection.
“At this time, there is limited supply of auto-injectors at wholesalers, distributors and at pharmacies,” said a release issued by Pfizer. “While we are working closely with our distributors to avoid long-term supply shortage at the store level, we expect a period of between two and four weeks of no inventory.”
While Pfizer and Health Canada work together to resolve the supply issue, the Canadian regulator has issued some advice for patients affected by the shortage.
“EpiPen auto-injectors expire on the last day of the month as indicated on the product packaging,” Health Canada said in a release about the shortage. “For example, if the product is marked as expiring in January it remains valid (not expired) until January 31. In general, it is recommended that individuals have more than one auto-injector with different expiry dates to avoid being in the situation of only having an expired auto-injector.”
This isn’t the first time that Pfizer-owned manufacturer of the EpiPen, Meridian Medical, has had a problem producing the devices. In 2017, Mylan issued a sweeping voluntary recall of potentially defective auto-injectors which were distributed to pharmacies in the US, Canada, New Zealand, Australia, Europe and Japan.
In all, 14 batches of the EpiPen were affected by the recall, representing over 80,000 medical devices. The devices were recalled over concerns that they may fail to activate in an emergency.







Join or login to leave a comment
JOIN LOGIN