Medtronic has announced that the FDA has granted 510(k) clearance for its MiniMed Go smart multiple daily injection (MDI) system.
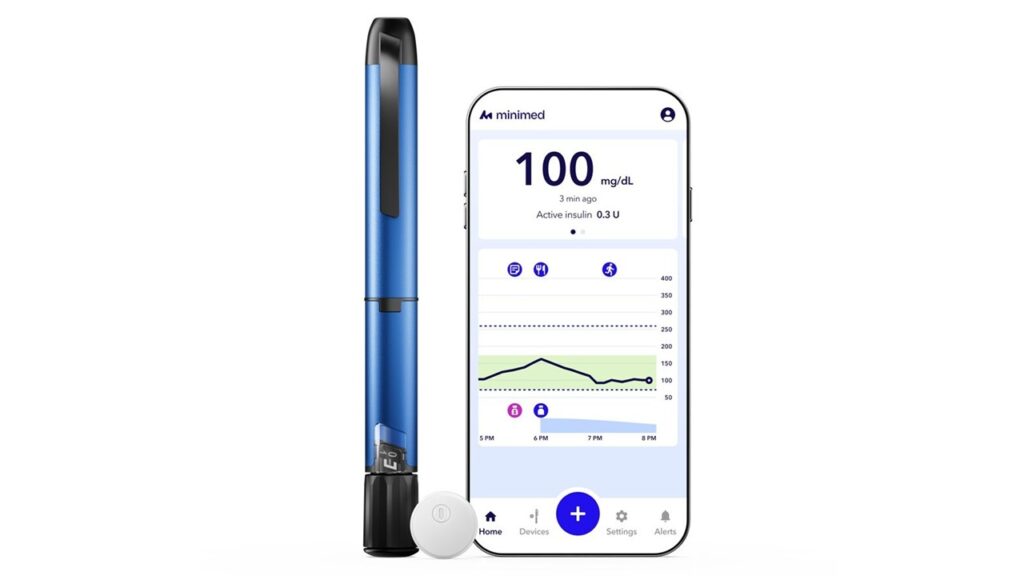
The MiniMed Go system is the first and only smart MDI solution that fully integrates personalized insulin dosing with continuous glucose monitoring (CGM) data in a single mobile app.
The platform connects Medtronic’s InPen smart insulin pen with the Instinct sensor developed by Abbott, providing real-time glucose insights, actionable alerts and data sharing tools that were previously limited to automated insulin delivery systems. The pen and sensor are linked via the MiniMed Go app.
Unlike traditional multiple daily injection regimens, where people with diabetes must manually calculate insulin doses and interpret glucose trends on their own, MiniMed Go offers several integrated functions, including missed dose alerts to reduce the risk of elevated glucose levels.
It also has built-in dose calculations to simplify complex insulin decisions, as well as actionable guidance following missed or incorrect injections. CareLink reporting to support collaboration between patients and healthcare teams is also available.
These features aim to lessen the daily cognitive burden of diabetes management and improve overall glycemic outcomes.
Related: Medtronic to Spin Off Diabetes Division Amid Strategic Refocus
Real-world data for the previous generation Medtronic Smart MDI system shows that users saw meaningful improvements in glycemic control when consistently responding to actionable alerts. Time in Range (TIR) increased from 55.7% to 67.2% when users addressed more than 75% of Missed Dose alerts within an hour, and to 71.5% when users addressed Correct High Glucose alerts with a bolus within an hour.
The FDA clearance covers individuals with insulin-requiring type 1 and type 2 diabetes aged seven years and older, as well as children aged two to six under adult supervision. Additional sensor compatibility, such as Medtronic’s Simplera sensor, is currently under review.
There are an estimated 15 million people worldwide relying on multiple daily injections, many of whom face stress and uncertainty around deciding correct insulin doses, particularly at mealtimes and during missed boluses. Even skipping a small number of injections weekly can negatively affect HbA1c and other key health outcomes.
MiniMed Go is designed to mitigate these challenges by automating key aspects of glucose interpretation and insulin decision-making.
“For too long, people using injections have carried the weight of diabetes management without access to the algorithms that make automated insulin delivery systems so powerful,” said Que Dallara, EVP and president of Medtronic Diabetes and CEO-designate of MiniMed. “MiniMed Go is designed to change that, bringing the smarts of an AID system to individuals who prefer an insulin pen.”
In December 2025, Medtronic commercially launched its MiniMed 780G system in the US, integrating it with Abbott’s Instinct sensor.
Medtronic plans to launch the MiniMed Go system commercially in the US later this spring.










Join or login to leave a comment
JOIN LOGIN