On Tuesday, the US Food and Drug Administration (FDA) approved a treatment for adults with a rare condition called thyroid eye disease or Grave’s eye disease. This is the first drug to be approved for this treatment.
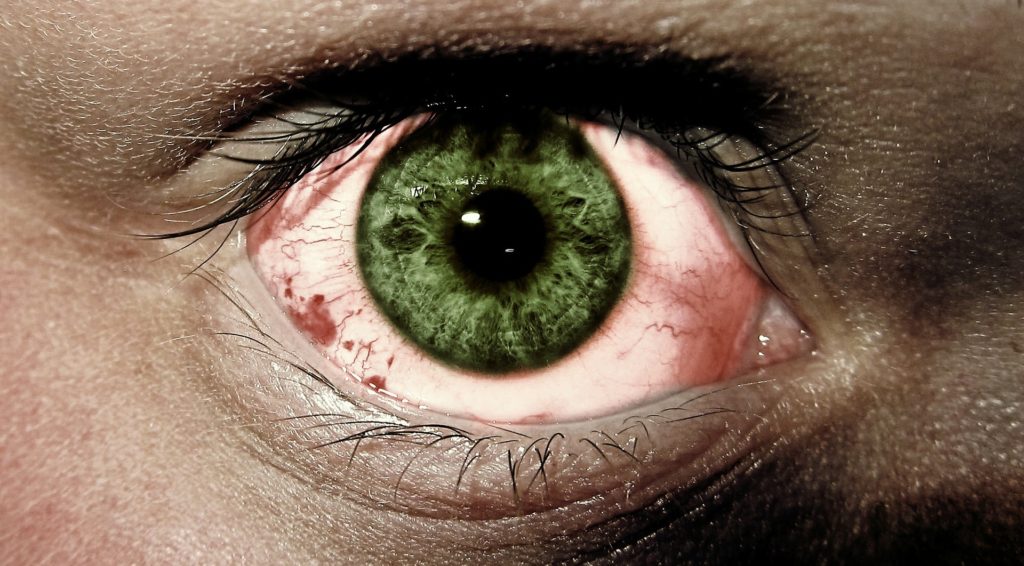
The drug is called Tepezza and it treats a rare condition behind the eye where the muscles and fatty tissues become inflamed and cause the eye to be pushed forward, making it bulge outwards. This bulging effect is known as proptosis.
“Today’s approval marks an important milestone for the treatment of thyroid eye disease. Currently, there are very limited treatment options for this potentially debilitating disease. This treatment has the potential to alter the course of the disease, potentially sparing patients from needing multiple invasive surgeries by providing an alternative, non‑surgical treatment option,” said Dr. Wiley Chambers, deputy director of the Division of Transplant and Ophthalmology Products in the FDA’s Center for Drug Evaluation and Research.
Thyroid eye disease is an autoimmune condition where the immune system attacks the thyroid glands and the body responds by secreting excess amounts of the thyroid hormone. This results in an excess number of hormones. Therefore, when the immune system attacks the tissues around the eye it causes the muscle to expand. This can cause eyes and eyelids to become red, swell, and cause discomfort. In some cases the swelling and stiffness make it difficult for the eye to move.
This disease affects approximately one million Americans each year. It also impacts an individual’s ability to perform important daily tasks, such as driving, because of the troubling ocular symptoms.
“Thyroid eye disease is a rare disease that impacts a small percentage of the population, and for a variety of reasons, treatments for rare diseases are often unavailable. This approval represents important progress in the approval of effective treatments for rare diseases, such as thyroid eye disease,” said Dr. Chambers.
The drug, Tepezza, was approved based on results from two studies that involved 170 patients with the active disease. They were chosen at random to either receive the drug or a placebo. In the study, the individuals that received the drug showed a two-millimetre reduction in proptosis compared to the subjects that received the placebo.
The FDA granted a Priority Review, Fast Track and Breakthrough Therapy designation for the drug. The drug also received Orphan Drug designation that provides an incentive to assist and encourage the development of drugs for this rare condition.







Join or login to leave a comment
JOIN LOGIN