The Burden of Alzheimer’s Disease
Almost everybody in the US has been affected by Alzheimer’s Disease. The Centers for Disease Control and Prevention (CDC) reports Alzheimer’s Disease as the sixth leading cause of death of Americans and fifth leading cause of death worldwide. In 2060, the CDC estimates that the number of people living with AD will exceed 14 million – exceeding the population of Los Angeles.
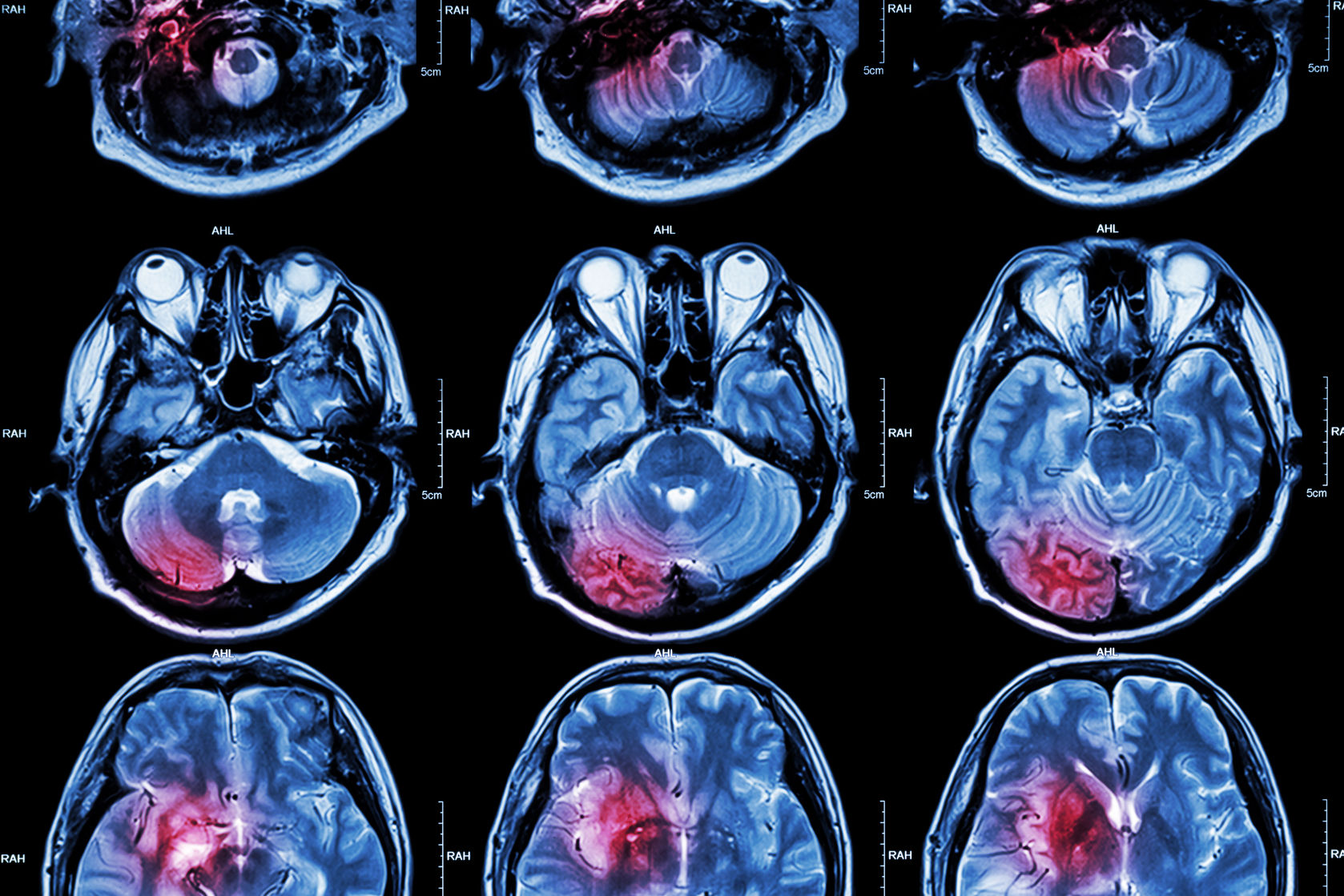
The neurodegenerative disease can lead to significant impairments in memory and cognition which severely impacts quality of life. An accumulation of beta amyloid plaques or neurofibrillary tangles comprising tau proteins is a widely accepted theory for the cause of Alzheimer’s Disease.
Each year, billions of dollars is poured into scientific research to advance our understanding of the disease, yet there are no effective strategies to prevent or treat Alzheimer’s Disease.
A Blood Test for the Early Detection of Alzheimer’s Disease
One of the ways to combat the Alzheimer’s Disease epidemic is to catch it early. Current diagnostic strategies are costly – an amyloid plaque PET scan – or invasive – a spinal tap to retrieve cerebrospinal fluid. While both methods can reliably detect the presence of amyloid plaques, there is a need for a minimally invasive, cost-effective test that can be broadly applied in clinical settings.
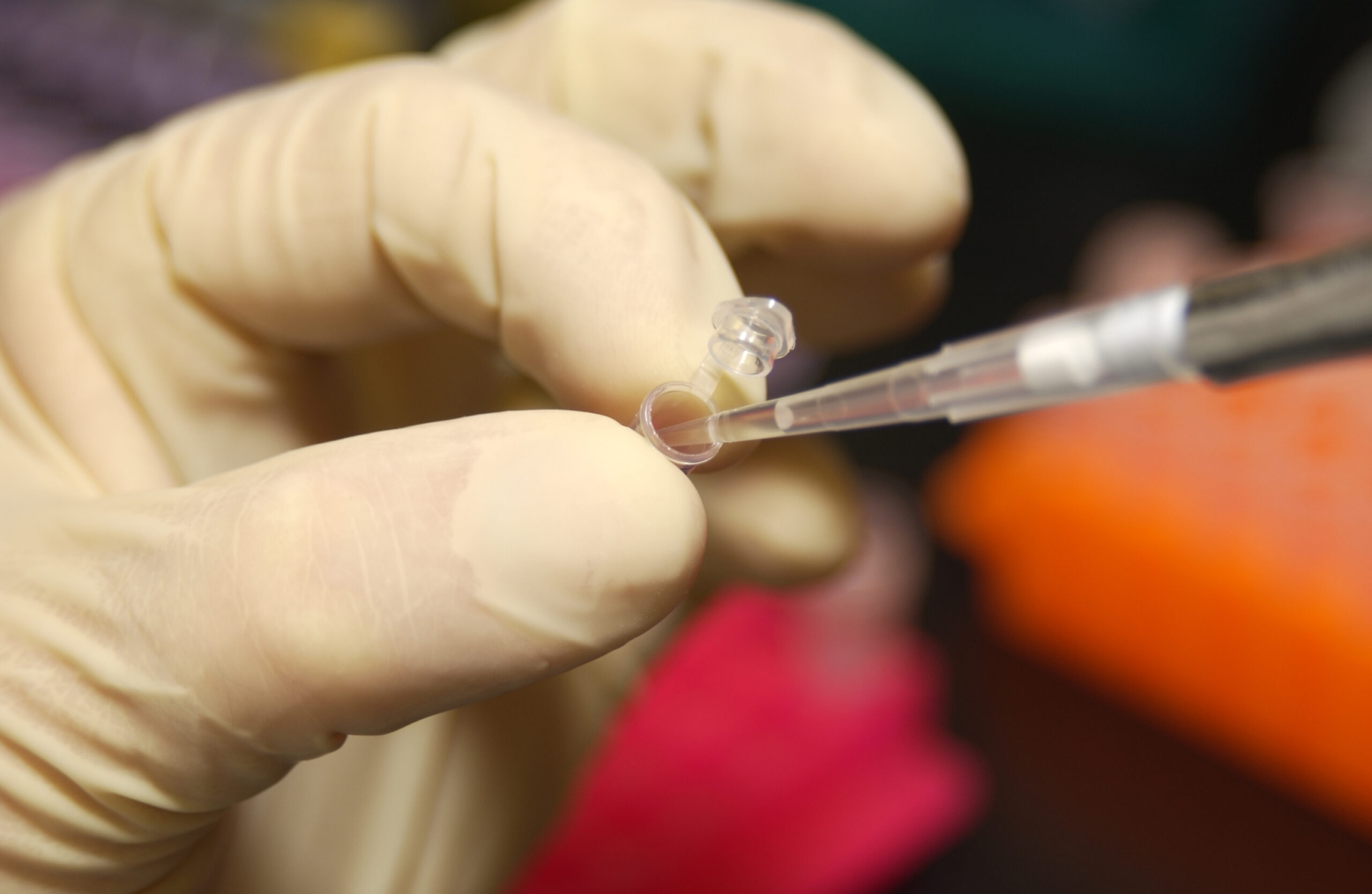
C2N Diagnostics, a biotechnology company which specializes in commercializing biomarker tests for neurodegenerative diseases, is developing a blood test that can screen for beta amyloid before a patient receives further diagnostic testing. The team recently received Breakthrough Device Designation from the US Food and Drug Administration (FDA).
“A simple, safe blood-based screening test would be the first step in a multistage Alzheimer’s Disease diagnostic process.” said C2N Diagnostics CEO, Dr. Joel Braunstein. “It would improve the speed and efficiency of the overall diagnostic process, and also afford significant cost savings to healthcare systems.”
Once blood is collected from the patient, a mass spectrometer determines the ratio of two beta amyloid isoforms – a lower ratio indicates brain amyloidosis. Identifying if people with minor memory loss have suspected Alzheimer’s Disease can be useful for screening patients for clinical trials.
“A screening test that pre-selects trial candidates that are more likely to have brain amyloidosis could decrease the screen failure rate and the overall time and cost of developing effective drugs for Alzheimer’s Disease,” said Dr. Ilana Fogelman, VP of Clinical and Regulatory Affairs at C2N Diagnostics.
If the test is approved by the FDA, it will become the first blood-based screening test for Alzheimer’s Disease diagnosis.
Reducing the Risk of Alzheimer’s Disease Early
Memory decline can be an early sign of Alzheimer’s Disease. Because it’s a progressive disorder, it is thought that early intervention can help prevent or slow cognitive decline.
Researchers from the University of South Florida (USF) have made a “pact” to slow cognitive decline to reduce the risk of Alzheimer’s Disease. In the new PACT (Preventing Alzheimer’s with Cognitive Training) trial, older adults will exercise their minds by playing computer games and solving puzzles over three years, during which researchers will track their cognition.
“This is a large primary prevention trial to examine if computerized cognitive exercises will reduce the risk of dementia,” said Dr. Elizabeth Hudak, Research Assistant Professor at USF Morsani College of Medicine. “It is the first of its kind study that will train adults on these exercises.”
Barriers to the Alzheimer’s Disease Drug Pipeline
Despite the plethora of Alzheimer’s Disease research, there are no commercially available cures. Scientists admit that our understanding of Alzheimer’s Disease is incomplete, but perhaps our “amyloid hypothesis” might be wrong – which could explain why many trials having been unsuccessful at finding a suitable drug target.
However, even after identifying a suitable drug target, the researchers must go through rigorous safety and efficacy testing before it hits the market. An estimated 54,000 participants are required to power an Alzheimer’s Disease clinical trial, a feat made almost impossible by strict inclusion criteria, length of study and generally low patient participation.
To overcome this barrier, researchers are hoping to repurpose market-approved drugs for the treatment of Alzheimer’s Disease. Research from the University of California San Diego published an article in the journal Cell Stem Cell detailing preliminary results involving an anti-HIV drug that indirectly targets tau protein, which is responsible for neurofibrillary tangles. By investigating pre-approved drugs, there is an opportunity for faster market approval.
Wide-Open Market for Alzheimer’s Disease Drugs
According to Data Bridge Market Research as cited by Journal Dairy, approximately 100 Alzheimer’s Disease drugs have been approved for Phase II and Phase III clinical trials in 2017. At this time, the Alzheimer’s Disease treatment market remains untapped, and is predicted to grow by 8 percent between 2018 to 2025.
The desire to meet an unmet clinical need and to seize market control has pharmaceutical companies racing to get their Alzheimer’s Disease drugs down the R&D pipeline. Among key market competitors are pharma giants Allergan, Novartis, Pfizer, Eli Lilly, Teva and Daiichi Sankyo.





Join or login to leave a comment
JOIN LOGIN