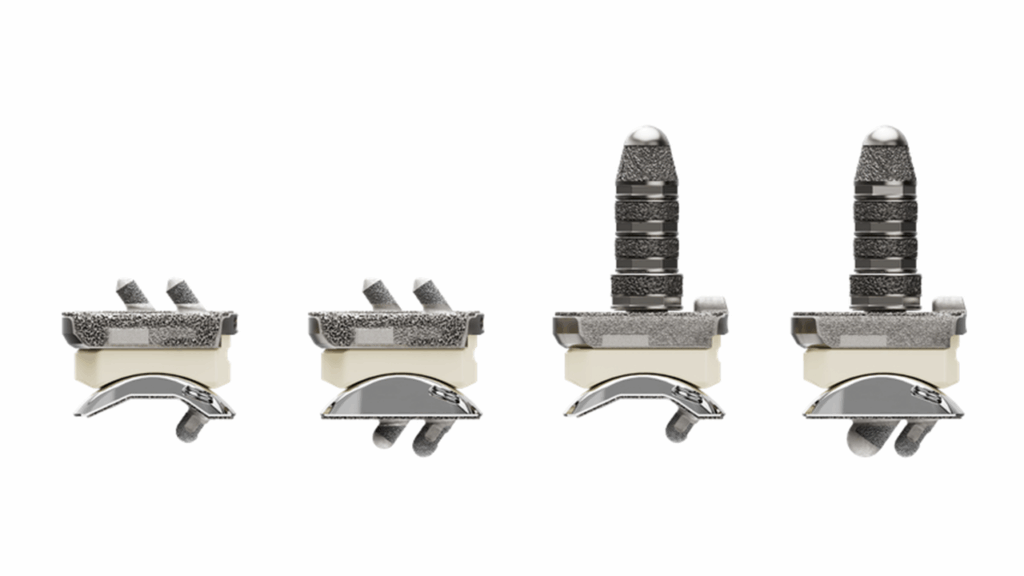
Stryker has received FDA clearance for its Incompass Total Ankle System, a new implant designed for patients with ankle joints damaged by severe rheumatoid, post-traumatic or degenerative arthritis.
These conditions are part of a broader global burden. Osteoarthritis, the most common form of arthritis, affects about 15% of adults over the age of 30 worldwide.
According to a 2023 Global Burden of Disease analysis, nearly a billion people may experience a form of osteoarthritis by 2050. And rheumatoid arthritis, while less prevalent, still affects approximately 0.5% to 1% of the global population and is a major contributor to joint damage and disability.
In advanced cases, both conditions can lead to joint erosion, chronic pain and loss of mobility, making surgical reconstruction a necessary option when conservative treatments fail.
Targeted at surgeons managing complex ankle reconstructions, the Incompass system is built to support surgical adaptability and improve procedural efficiency in total ankle replacement. The implant is intended to replace a severely damaged ankle joint in patients whose arthritis has not responded to nonsurgical treatments.
The system combines features from two of Stryker’s existing platforms — Inbone and Infinity — into a combined system that enables total ankle reconstruction. It offers a range of implants and instruments configured for anatomical alignment and fixation, incorporating Adaptis Boney Ingrowth Technology to support integration at the bone-implant interface.
Incompass was developed using Stryker’s Orthopaedic Modeling and Analytics (SOMA) platform, informed by data from more than 85,000 computed tomography (CT) scans and 100,000 clinical cases. This design foundation was further enhanced using Stryker’s Prophecy Surgical Planning System, arthritic ankle scans for preoperative planning tailored to patient anatomy.
According to Adam Jacobs, who leads Stryker’s Foot & Ankle business, the system was shaped by clinical insights and large-scale anatomical data. The Incompass is designed to help surgeons navigate diverse patient needs while maintaining procedural efficiency.
System enhancements include a redesigned alignment platform to support control across multiple planes of motion, updated implant holders and trial tools for improved intraoperative handling and instrumentation refinements intended to reduce surgical steps and operating room setup time.
The Incompass system expands Stryker’s Trauma and Extremities portfolio. The company has also been seeing continued growth across its Orthopaedics segment, with Q1 2025 data showing a 13.9% year-over-year increase in Trauma and Extremities sales, mainly driven by increased unit volume across extremity procedures.
Within the field of total ankle replacement and joint restoration, restor3d — a medical device company specializing in personalized orthopedic implants and 3D-printed joint systems — initiated a limited market release of its Aeros Modular Stem Total Ankle System in May 2025. The system adds to the expanding range of modular options being explored for end-stage ankle arthritis.
If you want your company to be featured on Xtalks.com, please email [email protected].








Join or login to leave a comment
JOIN LOGIN