Menarini Group has announced positive topline data from the Phase III BROADWAY and TANDEM clinical trials, evaluating obicetrapib and the fixed-dose combination of obicetrapib with ezetimibe.
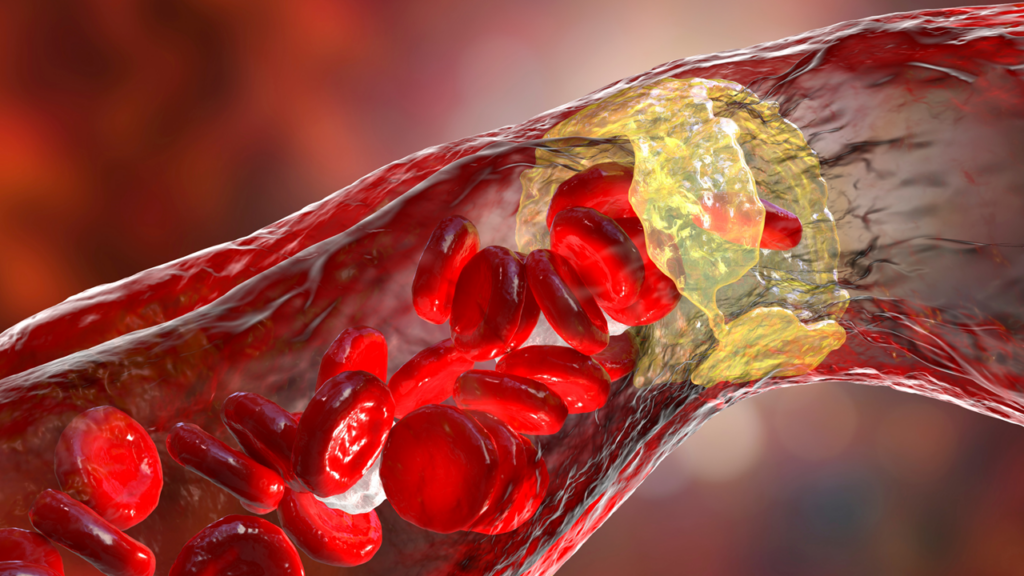
These trials aim to address a major challenge in cardiovascular disease: managing elevated low-density lipoprotein cholesterol (LDL-C) levels in patients at risk of heart disease.
While obicetrapib enhances cholesterol removal from the bloodstream, ezetimibe works by blocking cholesterol absorption in the small intestine.
Last month, NewAmsterdam Pharma shared positive topline data from the TANDEM trial, showing significant LDL-C reductions with the fixed-dose combination of obicetrapib and ezetimibe, with over 70 percent of patients reaching their target LDL-C levels. These results support global regulatory filings for the treatment. Menarini’s announcement builds on this, expanding on obicetrapib’s role in the broader Phase III program and its potential to enhance cardiovascular disease management.
The BROADWAY trial tested obicetrapib, a novel cholesteryl ester transfer protein (CETP) inhibitor, as a monotherapy in adult patients with heterozygous familial hypercholesterolemia (HeFH) or atherosclerotic cardiovascular disease (ASCVD).
These patients had not achieved adequate LDL-C reduction with maximally tolerated lipid-lowering therapies.
The trial’s primary endpoint — reduction in LDL-C — showed a 33 percent reduction in LDL-C with obicetrapib, compared to a two percent increase with placebo, reaching statistical significance (p<0.0001).
Additionally, the trial showed a 21 percent reduction in major adverse cardiovascular events (MACE), such as heart attack or stroke, favoring obicetrapib.
XTALKS WEBINAR: How Quality Management Drives Continuous Improvement for Life Sciences Organizations
Live and On-Demand: Wednesday, February 19, 2025, at 10am EST (3pm GMT/UK)
Register for this free webinar to learn about the shifting regulatory expectations surrounding quality and examine the latest trends shaping the life sciences sector.
Meanwhile, the TANDEM trial evaluated a fixed-dose combination of obicetrapib with 10 mg ezetimibe in a similar patient population.
This combination therapy demonstrated an even greater LDL-C reduction of 48.6 percent, significantly surpassing both obicetrapib alone and ezetimibe alone (p<0.0001).
These results suggest that combining obicetrapib with ezetimibe could offer an enhanced therapeutic approach for patients struggling to control their cholesterol levels.
Both trials were well-tolerated, with safety data comparable to placebo.
Treatment-emergent adverse events (TEAEs) were similar between the treatment and placebo groups, and no new safety issues emerged.
The trials also showed favorable effects on other cardiovascular risk factors, including non-HDL-C and lipoprotein(a) levels.
The BROADWAY and TANDEM trials are part of NewAmsterdam Pharma’s global Phase III program, which also includes the BROOKLYN and PREVAIL studies.
The BROOKLYN trial specifically targets patients with HeFH who struggle with LDL-C despite statin use, while the PREVAIL study is evaluating obicetrapib’s impact on cardiovascular outcomes in patients with a history of ASCVD.
These four studies collectively involve over 12,250 patients and represent a comprehensive effort to assess the efficacy and safety of obicetrapib across different patient populations.
Related: Rapiblyk (Landiolol): A New Fast-Acting Option for AFib in the ICU
In addition to obicetrapib, several other therapies are advancing in LDL-C management. Existing therapies such as Esperion Therapeutics’ Nexletol (bempedoic acid) and Nexlizet (bempedoic acid and ezetimibe), offer oral solutions that reduce LDL-C and cardiovascular risk, even for patients who don’t reach targets with statins.
Amgen’s Repatha (evolocumab) and Novartis’ Leqvio (inclisiran) are injectable proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors, with Leqvio offering a twice-yearly injection option. Unlike CETP inhibitors, PCSK9 inhibitors block a protein that regulates cholesterol levels.
Meanwhile, Merck is also testing an oral PCSK9 inhibitor, MK-0616, in late-stage trials.
Elcin Ergun, CEO of Menarini Group, highlighted that the Phase III results position obicetrapib as a potential first-in-class, low-dose oral treatment for high-risk cardiovascular patients. The company plans to further evaluate obicetrapib in the ongoing PREVAIL trial to confirm its long-term benefits in reducing cardiovascular risk.











Join or login to leave a comment
JOIN LOGIN