The FDA has cleared Medtronic’s OmniaSecure defibrillation lead. This marks a key development for patients indicated for an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D).
An ICD monitors for dangerously fast arrhythmias and delivers shocks to restore normal rhythm. In contrast, a pacemaker regulates slow heartbeats.
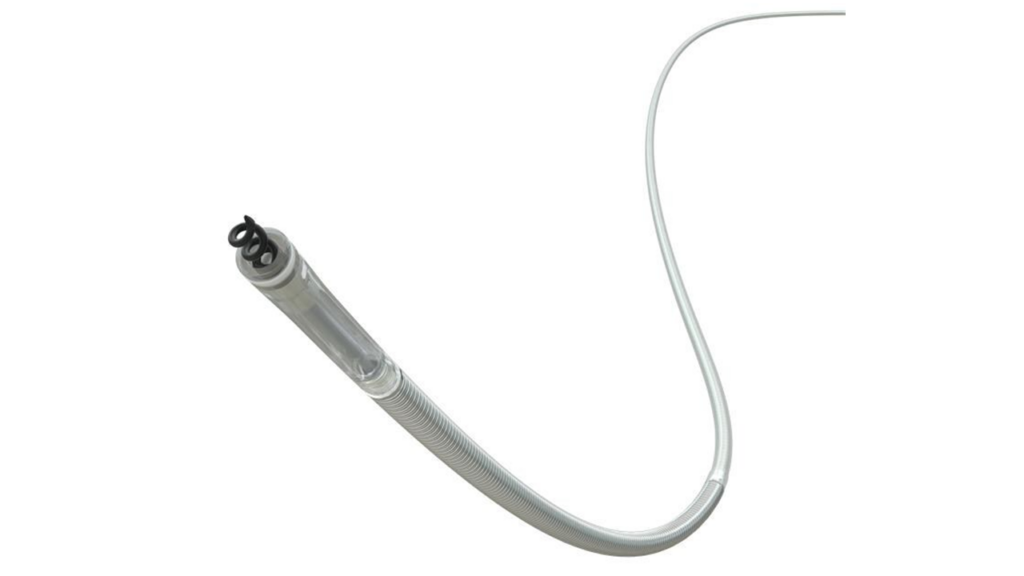
Medtronic designed the OmniaSecure lead for right ventricular placement. It is now the world’s smallest-diameter defibrillation lead. OmniaSecure is indicated for stimulation in the right ventricle for adults and adolescents aged 12 and older, including those with smaller heart structures.
The OmniaSecure lead connects to an implanted defibrillator. It monitors heart rhythms and delivers therapy when it detects life-threatening arrhythmias such as ventricular fibrillation or tachycardia. Over a patient’s lifetime, defibrillation leads must flex with millions of heart contractions. This makes durability a key design consideration in systems like OmniaSecure.
Traditional, larger-diameter leads may raise the risk of complications such as venous occlusion or tricuspid valve regurgitation. Medtronic reduced the lead’s size to 4.7 French (approximately 1.6 mm) to lower these risks while maintaining performance. The new design builds on the established platform of the SelectSecure Model 3830 pacing lead.
Beyond its cleared use, OmniaSecure is also being studied for placement in the left bundle branch (LBB) area — closer to the heart’s natural conduction system. The OmniaSecure lead remains investigational for LBB placement and is not yet FDA-approved for this indication.
According to Dr. Pugazhendhi Vijayaraman, cardiac electrophysiologist at Geisinger Wyoming Valley Medical Center, this approach may enable physiologic pacing by activating native conduction fibers, leading to more synchronized heartbeats compared to traditional pacing methods.
Clinical Trial Results for OmniaSecure
Researchers presented preliminary findings from the LEADR LBBAP study — which supported OmniaSecure’s approval — at the Heart Rhythm 2025 conference. The study, which enrolled approximately 300 participants across 24 global sites, showed encouraging results.
Defibrillation testing at implant was successful in 100% of the first 162 patients, exceeding the study’s prespecified efficacy goal of 88%. Among the first 193 patients, the OmniaSecure lead was successfully implanted per protocol in 95.8% of procedures. There were no major complications, such as early helix or lead fracture, system revision or death, reported.
These findings build on earlier results from the global LEADR pivotal trial. That study demonstrated that OmniaSecure met its primary safety and effectiveness endpoints for right ventricular placement and exceeded prespecified performance goals.
Medtronic is continuing to follow patients in the LEADR LBBAP study, with ICD-indicated patients followed for 3 months and CRT-D-indicated patients followed for 6 months post-implant.
At the same Heart Rhythm 2025 conference, Medtronic also presented new data from its investigational Affera ablation platform for atrial fibrillation. In a one-year study, Sphere-360 — a single-shot catheter designed to isolate pulmonary veins using pulsed field ablation — demonstrated freedom from arrhythmia recurrence in 88% of patients. It also achieved durable pulmonary vein isolation in 98% of targeted veins.
Late last month, Medtronic shared five-year results from the Evolut Low Risk trial, reporting a numerically lower mortality rate with transcatheter aortic valve replacement (TAVR) compared to surgery (15.5% vs. 16.4%, respectively). Patients treated with Evolut also showed better valve function at five years, including significantly wider valve openings and lower pressure gradients.
As global demand for ICDs continues to grow — with the market valued at $3.81 billion in 2023 and projected to expand steadily through 2030 — innovations like OmniaSecure aim to make therapy delivery more precise and reduce complications tied to larger, traditional leads.
If you want your company to be featured on Xtalks.com, please email [email protected].










Join or login to leave a comment
JOIN LOGIN