Regeneron Pharmaceuticals’ macular degeneration drug Eylea has met its primary endpoint in a Phase III clinical trial of non-proliferative diabetic retinopathy. The injectable drug was associated with a two-step or better improvement from baseline on the Diabetic Retinopathy Severity Scale (DRSS) in 58 percent of patients treated with Eylea, compared to 6 percent of those in the placebo group.
“This is the first time a therapy has demonstrated it can reverse disease progression in patients with moderately severe to severe non-proliferative diabetic retinopathy without diabetic macular edema, in a trial specifically designed to study this population,” said Dr. George D. Yancopoulos, President and Chief Scientific Officer of Regeneron. “Patients in the trial continue to be evaluated to determine if Eylea can prevent progression to neovascular vision-threatening complications or diabetic macular edema. We look forward to sharing one-year results later this year.”
The PANORAMA trial is an ongoing two-year trial that has currently enrolled just over 400 patients. The current data is from a 24-week subset of the study in which patients in the treatment arm received an average of 4.4 Eylea injections. The study investigators noted that while one case of mild intraocular inflammation was identified in a patient being treated with Eylea, the rate of adverse events was consistent with previous studies.
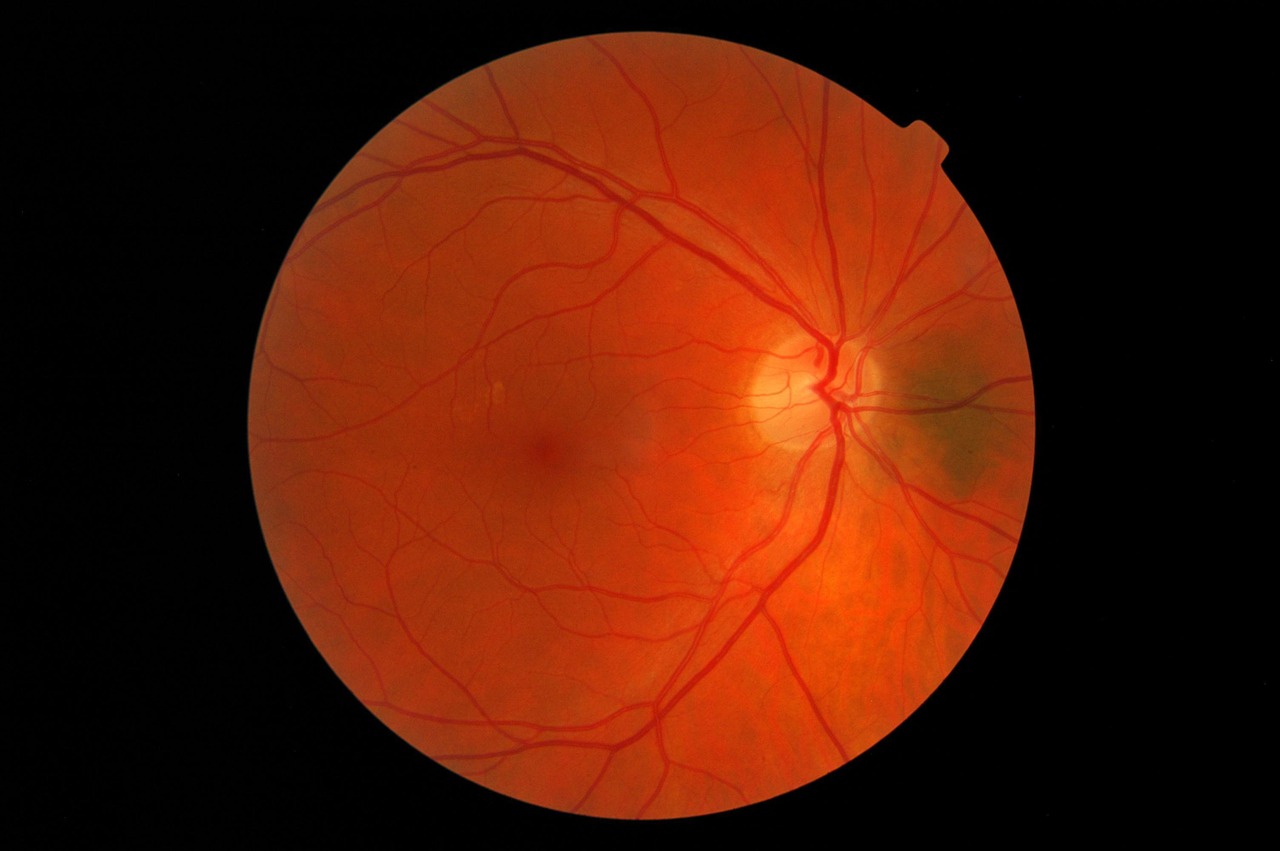
Diabetic retinopathy affects about eight million people in the US and is caused by a lack of blood glucose control in individuals with diabetes. In the early stages of the disease, blood vessels in the retina sustain diabetes-associated microvascular damage which generally manifests itself as asymptomatic non-proliferative diabetic retinopathy.
As non-proliferative diabetic retinopathy worsens, patients may start to develop diabetic macular edema (DME) which can lead to vision loss. Eylea is currently approved by the FDA to treat patients with non-proliferative diabetic retinopathy with DME, however the 560,000 patients without DME don’t have this same treatment option.
The ongoing PANARAMA trial will assess both eight-week and 16-week dosing schedules in patients with diabetic retinopathy without DME. The co-primary endpoint will also assess the number of patients who see a two-step or greater improvement on the DRSS score after 52 weeks.
According to Regeneron, the results from this Phase III trial will be used to support a supplemental Biologics License Application (sBLA) to be submitted to the FDA in late 2018.







Join or login to leave a comment
JOIN LOGIN