Clinical trials for atherosclerotic cardiovascular disease (ASCVD) are increasingly turning to advanced imaging to uncover what conventional tools might miss — particularly perivascular inflammation. While traditional scoring systems like calcium burden offer structural insights, they often fail to detect early biological activity that could predict future cardiovascular events.
According to a report, the ASCVD market reached $24 billion across the US, EU4, UK and Japan in 2024 and is projected to grow to $32.3 billion by 2035 — an expansion fueled by the rising use of statins, antiplatelet therapies and minimally invasive interventions, as well as growing interest in gene and regenerative therapies.
Yet as the disease becomes better understood, so too must trial strategies evolve.
In a recent webinar, Dr. Charalambos Antoniades, Co-Founder and Chief Scientific Officer of Caristo Diagnostics, joined Medpace experts Dr. Rina Ariga, Medical Director, and Dr. Andrea Flannery, Director of Clinical Trial Management at Medpace, to discuss how imaging biomarkers like the fat attenuation index (FAI) and emerging techniques such as radiotranscriptomics are changing how ASCVD trials are designed and interpreted.
Read on to learn how this expert panel is advancing imaging strategies that improve risk prediction, clarify biological mechanisms and support operational planning in cardiovascular trials.
Rethinking Inflammation Detection in Cardiovascular Risk
Dr. Antoniades opened his talk with a fundamental question in cardiovascular prevention: should we pursue a cost-effective, one-size-fits-all approach like the poly-pill, or invest in precision medicine that targets the right treatments to the right patients?
While current practice still relies heavily on risk-factor-based calculators, Dr. Antoniades argued that meaningful implementation of precision medicine requires integration of genetics, plasma biomarkers and imaging biomarkers.
He explained that coronary artery disease affects about 6.5% of the population, typically identified when patients develop symptoms. However, nearly 60% of heart attacks occur in individuals without symptoms or detectable obstructive disease. This underscores the need to detect disease earlier, even before significant plaque formation, by identifying inflamed arteries and vulnerable plaques.
Coronary CT angiography (CCTA) now serves as the first-line test in many cases of chest pain. Findings from the ORFAN study — a prospective study of 40,000 patients followed for three years after undergoing CCTA — showed that only 18% had obstructive disease, yet two-thirds of heart attacks occurred in patients without obstruction. That insight changed the thinking around inflammation as a therapeutic target and a diagnostic gap.
He cited several clinical trials — CANTOS, LoDoCo2 and COLCOT — which confirmed that targeting inflammation reduces cardiovascular events, leading to colchicine’s FDA approval for cardiovascular risk reduction in 2023.
Still, a key challenge remains: identifying the subset of patients with inflamed coronary arteries. Plasma biomarkers reflect systemic inflammation, not localized arterial inflammation.
To bridge that gap, Dr. Antoniades and his team at the University of Oxford studied how inflamed arteries alter the surrounding perivascular adipose tissue (PVAT). When arteries are inflamed, PVAT cells shrink and lose lipid content — a dynamic change visible even before plaque forms (Figure 1).

To make this insight clinically actionable, his group developed FAI — a CT-based “virtual biopsy” that reveals inflammation by analyzing the attenuation patterns of PVAT.
Normal, non-inflamed arteries appear yellow, whereas inflamed regions appear red. In some cases, patches of blue indicate fibrosis and edema linked to specific inflammatory pathways.
Dr. Antoniades spoke about a photon-counting CT research facility (Figure 2) integrated with a cardiac catheterization lab (cath lab) to validate FAI and support its clinical utility compared to intravascular imaging.

The team refined the method further, developing an FAI score that adjusts for anatomical and technical variables, allowing broad application across coronary vessels. This score has been regulator-approved as a biomarker of coronary inflammation in Europe, the UK and Australia, and was incorporated into a European Society of Cardiology (ESC) clinical consensus statement on coronary inflammation imaging.
Validating FAI: What the Data Show
Dr. Antoniades outlined how large-scale studies have established FAI’s value as a cardiovascular imaging biomarker.
In the CRISP-CT study of 4,000 patients in the US and Germany, FAI improved risk prediction beyond traditional markers like calcium scores and plaque burden. It increased the C-statistic — a measure of a model’s ability to differentiate between patients with and without events — by 5.4 to 7.5 points.
To assess generalizability, his team launched the ORFAN study, aiming to enroll 250,000 patients undergoing coronary CT. As of mid-2025, over 210,000 patients have been recruited.
Early findings from the UK arm of the ORFAN study revealed that patients with FAI scores above the 75th percentile were at markedly higher risk for adverse outcomes. Specifically, those without obstructive coronary disease faced a tenfold greater risk of cardiac death, while those with obstructive disease had a fivefold increase. Inflammation scores also correlated with a 3.5 to 5.3 times higher likelihood of non-fatal myocardial infarction across all three coronary arteries. Additionally, high FAI values were linked to a greater incidence of ischemic heart failure — even in patients without prior myocardial infarction.
These outcomes were observed even in patients with no visible plaque or a calcium score of zero. Data from ORFAN further reported hazard ratios exceeding 10 for cardiac death in patients with minor or no plaque but high FAI, reinforcing the biomarker’s independent prognostic value.
Dr. Antoniades noted that FAI also helps monitor treatment effects. In patients with psoriasis, biologic therapies such as anti-TNF and anti-IL agents have led to notable reductions in FAI. – Post-STEMI (ST-elevation myocardial infarction) patients — those recovering from a severe heart attack caused by a fully blocked artery — showed significant FAI decreases within six months. However, 20% remained non-responders, a group that could likely benefit from novel therapeutics.
Statin therapy has shown a 30% to 40% reduction in FAI within one year, sustained for at least three years. In a 12-week Phase II randomized clinical trial, orticumab, a monoclonal antibody targeting oxidized LDL, significantly lowered FAI in all three coronary arteries — a promising indicator for Phase II trials.
Dr. Antoniades also pointed to an observational study in breast cancer patients receiving radiotherapy. While calcium scores rose — typically considered a negative signal — inflammation levels dropped across all coronary arteries. He suggested this may reflect plaque stabilization after tumor removal rather than elevated cardiovascular risk.
Collectively, these findings point to FAI as a promising early marker that could add value alongside conventional imaging and clinical evaluations, particularly in identifying patients who may not be flagged through standard measures.
Toward Smarter Trials: Radiotranscriptomics and AI Integration
To refine patient selection for cardiovascular trials, Dr. Antoniades emphasized the need to help personalize cardiovascular risk prediction. Dr. Antoniades introduced CaRi-HEART, an AI-powered platform that combines CCTA with clinical and biological data to estimate absolute cardiac risk.
Trained on cohorts in the US and Germany and validated in the UK, CaRi-HEART achieved a C-index of 0.84 for predicting cardiac mortality. In the UK’s National Health Service’s (NHS) use, the tool influenced treatment decisions in 45% of patients — prompting statin initiation in 24%, dose increases in 13% and referrals in 8%. These shifts underscore its potential to guide trial enrichment and target patients most likely to benefit from intervention.
To push this further, Dr. Antoniades’ team developed the Heart, Vessels and Fat (HPF) Library — a radiotranscriptomic dataset linking visible fat changes on CT to inflammatory signals identified via RNA sequencing. This work aims to connect imaging features with underlying biology, supporting more precise patient selection and early efficacy insights in clinical trials.
He also pointed to a real-world application: an FAI-based inflammation score created during the COVID-19 pandemic to identify patients likely to respond to dexamethasone. Deployed across US and UK hospitals, the tool demonstrated how image-derived biomarkers can inform urgent treatment decisions — and may pave the way for broader use in chronic disease management and drug development.
“In the future, it [FAI] could potentially be used as a complementary or companion diagnostic for cardiovascular outcome trials,” he concluded.
Why Imaging Biomarkers Matter in CV Trials
Imaging is more than a diagnostic tool — it is fundamental to how cardiovascular trials are designed, enriched and interpreted. Dr. Ariga cited FAI as an example of how imaging biomarkers help visualize and quantify biological changes (Figure 3), often earlier than clinical events like myocardial infarction.

“FAI is an example of how imaging is no longer just a diagnostic tool,” she said, “it’s becoming an integral part of how we design and interpret cardiovascular trials.”
She explained that imaging biomarkers allow for non-invasive visualization of changes in disease activity, from plaque burden to inflammation. These biomarkers can provide early readouts on treatment efficacy, support mechanism of action studies and help shorten trial durations.
Imaging can also stratify patients, identifying those most likely to show a treatment effect. This boosts statistical power and may reduce sample size or trial duration. Regulators are increasingly open to the use of imaging biomarkers, both as surrogate endpoints and as tools for risk enrichment and prognosis.
Infrastructure and Operational Capabilities
Dr. Ariga emphasized that scientific rationale alone isn’t enough — successfully integrating imaging into trials requires operational expertise. This includes managing vendor relationships, ensuring protocol compliance and maintaining high-quality image data across multiple trial partners (Figure 4).

She noted that Medpace supports cardiovascular trials using a range of imaging modalities, including CT, ECHO, MRI, PET and invasive coronary angiography. Their integrated cardiovascular imaging core lab features imaging-certified cardiologists and technical teams skilled in working with both in-house and external imaging vendors.
“Whether imaging is handled in-house or through a sponsor-preferred external core lab,” she said, “experience is needed to navigate the imaging vendor relationship to oversee compliance.”
Imaging Considerations in Trial Design
Dr. Ariga outlined a set of questions her team considers when designing imaging-inclusive trials (Figure 5). Selecting the appropriate imaging modality requires aligning it with the biological question.

The modality must also be reproducible across multiple centers, validated and capable of reflecting meaningful outcomes like symptom improvement or survival.
Timing of imaging is equally important — it must coincide with expected biological changes without overburdening patients or sites. For example, while more frequent imaging may offer deeper insights, it can increase radiation exposure and costs.
Dr. Ariga emphasized that tight imaging inclusion criteria can also affect diversity. Patients with kidney issues, for instance, may be excluded from contrast CT-based trials.
Standardization and Imaging Oversight
Dr. Ariga highlighted the importance of rigorous statistical planning and operational execution (Figure 6). Imaging endpoints must be assessed for sensitivity and variability, especially in adaptive trial designs. Interim analyses should be pre-specified to maintain scientific validity.

At the site level, high-volume imaging centers and standardized protocols are key to ensuring consistent image quality.
“We do that through adherence to standardized imaging protocols,” she said, emphasizing that robust operational infrastructure is key to translating imaging biomarkers into actionable trial outcomes.
Optimizing Site Selection with Data-Driven Feasibility
Dr. Flannery shifted the conversation toward operational readiness in cardiovascular trials, focusing on how to embed imaging excellence from the ground up.
“First and foremost, it is essential to select the best sites for the success of any clinical trial,” she said. Site selection must be more than a routine box-checking exercise. It should be a data-driven, experience-refined process based on imaging experience and metrics for a particular site.
Feasibility should account for prior trial performance, patient population fit and site familiarity with imaging standards. Drawing from platforms like imaging core lab databases, central labs and historical study metrics allows for more accurate site targeting — especially when imaging endpoints are involved.
Dr. Flannery also underscored the importance of early collaboration. She recommended this team include expert team members such as medical leads, operational leads, regulatory specialists, imaging experts and other key personnel from the sponsor, CRO and any relevant vendors. A cross-functional team ensures that site feasibility assessments reflect real-time clinical requirements and demands..
She described a “one-team approach” as essential to trial continuity, spanning from site qualification through imaging acquisition and quality oversight (Figure 7). This includes verifying technician availability, understanding interdepartmental scheduling logistics and preparing contingency plans in case imaging needs to be shifted to a backup facility. Training isn’t one-and-done, she added; it should persist across trial stages to reinforce standardization.
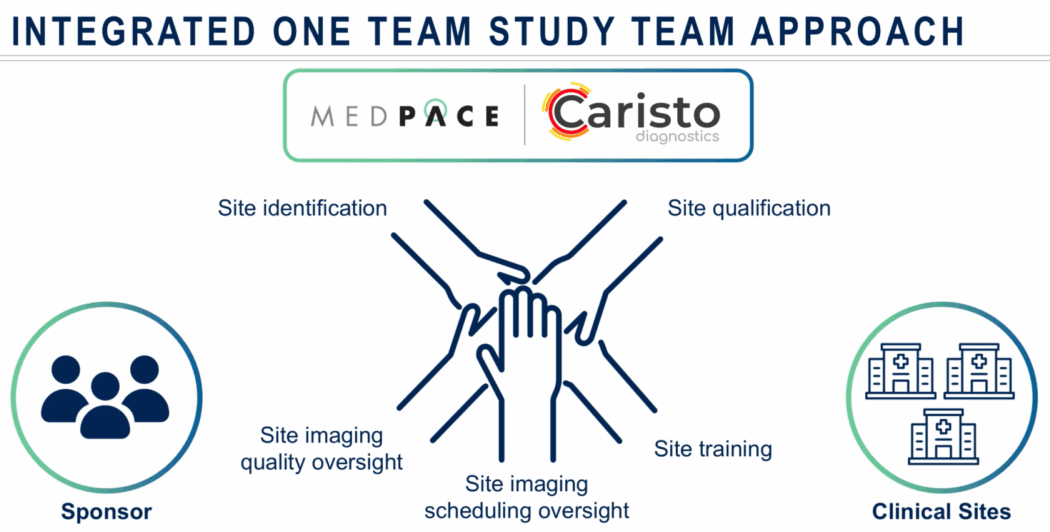
During trial activation and enrollment, clinical trial teams — including CROs and imaging vendors — must align on imaging manual requirements, technician delegation and data turnaround expectations (Figure 8). Tools like automated dashboards can flag early deviations or scheduling inconsistencies before they impact study quality and data integrity.

“Finally, and most importantly,” Dr. Flannery said, “is embedding quality risk management at the start and throughout the lifecycle of the study.”
She concluded that a robust oversight framework — with pre-defined triggers for retraining, centralized monitoring and integration across vendors and sponsors — is no longer optional.
As cardiovascular trials become increasingly complex, consistent oversight across all stakeholders could become critical to protecting data integrity and patient safety.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.







Join or login to leave a comment
JOIN LOGIN