Update (January 23, 2019):
Merck announced that it will ship another 120,000 doses of their experimental Ebola vaccine, on top of 100,000 doses that already arrived, to combat the growing outbreak in the Congo. While the vaccine has yet to receive regulatory approval, over 600,000 people have already received the vaccine, according to the World Health Organization.
Originally published December 11, 2018:
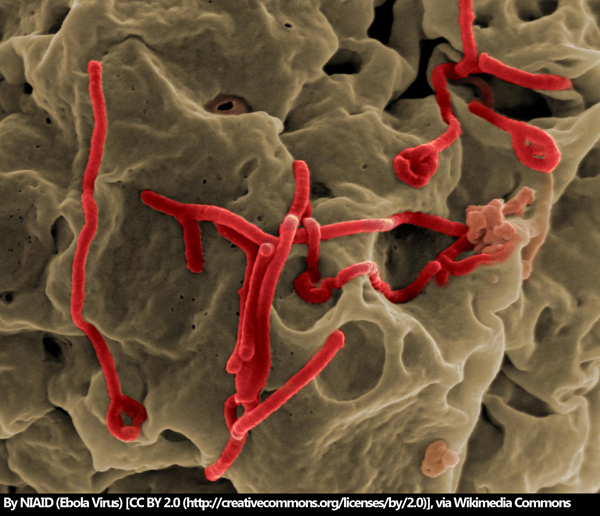
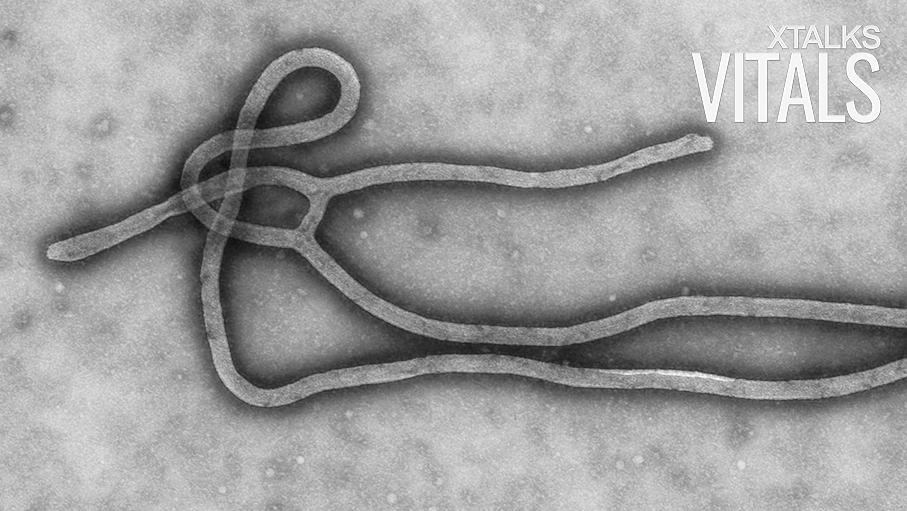
As of December 4, 2018, the number of Ebola cases in the Democratic Republic of the Congo has hit 458, according to the World Health Organization (WHO). After the first probable case was reported in late April 2018, the rapid spread of the infection to 11 health zones across the country is alarming. What’s more, there is a growing concern that the world supply of Ebola virus vaccines is running low.
The outbreak of 2014 resulted in over 11,000 Ebola deaths across Guinea, Liberia and Sierra Leone and cost an estimated $4.3 billion, according to the Centres of Disease Control and Prevention. The first Ebola vaccine was discovered and developed by the Public Health Agency of Canada, later to be licensed to NewLink Genetics, then to Merck. Since the outbreak, Merck has continued the clinical development of a new Ebola vaccine, the rVSV-ZEBOV vaccine, pursuing accelerated regulatory approval by the Food and Drug Administration (FDA).
Over the last few months, Merck Ebola vaccines have been given to those with close contact with Ebola cases (family members and healthcare providers) and even contacts of contacts (ambulance drivers and funeral attendees). This ring vaccination approach intends to stop transmission of the virus before it radiates out to neighboring countries. At this rate of vaccination, the current stockpile of 300,000 doses does not appear to be sustainable.
“When you consider that the global…stockpile is 300,000 doses and Beni itself has a population of 400,000 and Butembo a million,” said Dr. Peter Salama, Deputy Director-General of Emergency Preparedness and Response for WHO, to STATNews, “you can imagine that when people are proposing large-scale geographical vaccination that becomes extremely impractical.”
The limiting factor is converting Merck’s bulk supply of the vaccine into vials, which can take several months, according to a Merck spokeswoman. The National Institute of Allergy and Infectious Diseases is supporting GSK, Janssen Pharmaceutical Companies and Profectus Biosciences Inc. in the development of other Ebola vaccines.
The Democratic Republic of the Congo is simultaneously experiencing cholera, vaccine-derived poliomyelitis and malaria epidemics, slowing the response to the Ebola outbreak. While the risk remains high at the national and regional levels, the WHO is confident that the outbreak can be contained and resolved.




Join or login to leave a comment
JOIN LOGIN