The heart and vascular medication market continues to be a critical focus area for pharma companies, with billions of dollars in sales generated annually. These medications play a vital role in managing conditions such as heart failure, atrial fibrillation, stroke prevention and cholesterol reduction, improving patient outcomes and quality of life worldwide.
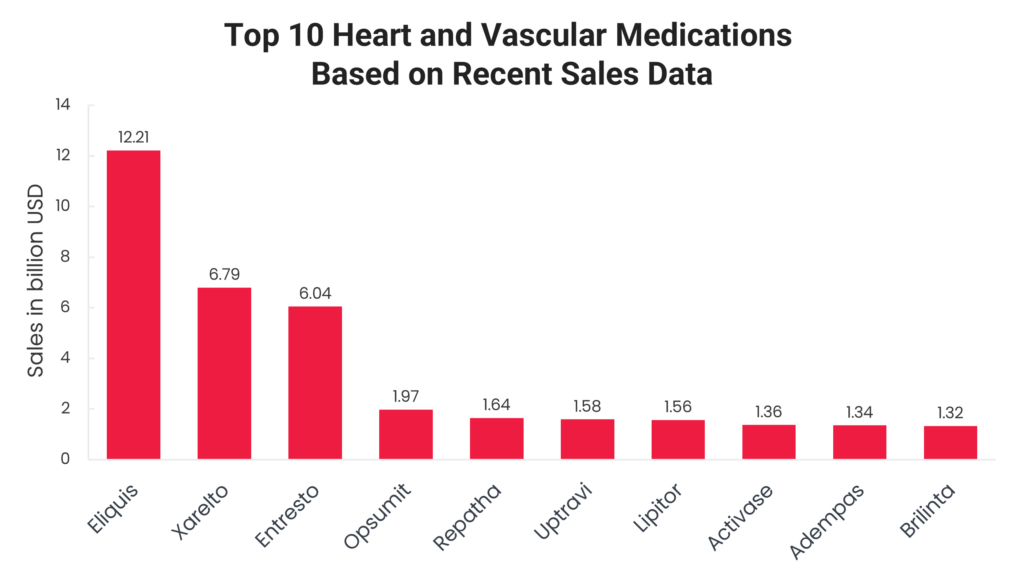
In this blog, we take a closer look at the top 10 heart and vascular medications based on recent sales data. From blockbuster anticoagulants like Eliquis (apixaban) and Xarelto (rivaroxaban) to life-saving treatments such as Entresto (sacubitril/valsartan) and Brilinta (ticagrelor), each of these drugs has made a significant impact in the cardiovascular space. Their widespread adoption is driven by strong clinical evidence, broad FDA-approved indications and continued advancements in treatment protocols.
As the demand for cardiovascular treatments grows amid rising global incidence rates of heart-related conditions, pharma companies strive to maintain their competitive edge through innovation, strategic partnerships and market expansion efforts. However, challenges such as patent expirations, generic competition and pricing pressures continue to shape the market landscape.
Read on to explore the leading heart and vascular medications, analyzing their sales performance from full-year 2023, key clinical benefits and market dynamics that have contributed to their success.
1. Eliquis (Apixaban)
Eliquis 2023 sales: $12.21 billion
Company/developer: Bristol Myers Squibb (BMS), Pfizer
Date of first FDA approval: December 28, 2012
FDA-approved indications: Stroke prevention in atrial fibrillation; venous thromboembolism (VTE) prevention; orthopedic surgery; VTE treatment
Price of Eliquis: The list price for a 30-day supply of Eliquis is $594.
Why it sold so well: Sales of BMS’s blockbuster blood thinner, Eliquis, increased by 4% year-over-year, maintaining its position as the leading oral anticoagulant globally. Partnered with Pfizer, the drug continues to show strong market performance. During a fourth-quarter earnings call in early 2024, BMS’s chief financial officer, David Elkins, expressed confidence in continued “strong growth” within the US market in 2024. In 2023, Pfizer generated $6.75 billion in revenue from Eliquis, reflecting a similar 4% growth. Pfizer retains full commercial rights to the drug in select smaller markets.
Eliquis remains the top contender in the factor Xa inhibitor category, outperforming Bayer and Johnson & Johnson’s Xarelto. Its success is largely attributed to strong adoption in the non-valvular atrial fibrillation market and its clinical advantages over the traditional anticoagulant warfarin. Unlike warfarin, Eliquis does not require regular monitoring and offers a lower risk of major bleeding, which has further driven its widespread acceptance.
The patent for Eliquis in the US was initially set to expire in February 2023 but received an extension from the US Patent and Trademark Office, now lasting until November 2026. Although the FDA approved generic versions from Micro Labs Limited and Mylan Pharmaceuticals Inc. (now Viatris) in 2019, BMS and Pfizer reached settlements with both companies, delaying their market entry until April 1, 2028. However, generic versions have already launched in countries such as Canada and the UK, contributing to a 10% decline in Eliquis sales outside the US last year.
In 2023, Eliquis was selected as one of the first 10 drugs subject to Medicare price negotiations under the Inflation Reduction Act (IRA), which will take effect in 2026. The drug’s annual cost in the US, which stands at $7,100, has come under scrutiny, with US Senator Bernie Sanders criticizing the pricing during a Senate committee hearing in February 2024. Sanders questioned BMS CEO Chris Boerner on why the drug is priced at $900 per year in Canada while its manufacturing cost is estimated at just $18.
2. Xarelto (Rivaroxaban)
Xarelto 2023 sales: $6.79 billion
Company/developer: Bayer, marketed in collaboration with Janssen Pharmaceuticals (a Johnson & Johnson company)
Date of first FDA approval: July 1, 2011
FDA-approved indications: Reducing the risk of stroke and systemic embolism in individuals with atrial fibrillation; treatment and reduction in the risk of recurrence of deep vein thrombosis (DVT); treatment and reduction in the risk of recurrence of pulmonary embolism (PE); prophylaxis of DVT which may lead to PE in patients undergoing knee or hip replacement surgery; VTE in acutely ill medical patients at risk for thromboembolic complications who are not at high risk of bleeding; reducing the risk of major cardiovascular events such as stroke, heart attack and amputation in individuals with coronary artery disease (CAD) and peripheral artery disease (PAD)
Price of Xarelto: $542 for a 30-day supply
Why it sold so well: Xarelto is an oral anticoagulant classified as a direct factor Xa inhibitor. It offers an effective and safe solution for preventing and treating blood clot disorders, providing a significant advantage through its once-daily oral dosing. This simplified regimen makes it more convenient compared to traditional therapies such as warfarin, which often require frequent dosing and monitoring.
The drug’s ongoing success is largely attributed to its broad range of FDA-approved indications, including stroke prevention in patients with atrial fibrillation, treatment and prevention of DVT and PE, and reducing major cardiovascular events in individuals with chronic CAD or PAD. These approvals contribute to its extensive use across a diverse patient population.
Xarelto continues to be supported by robust clinical evidence. Key studies, such as VOYAGER PAD and PIONEER AF-PCI, have highlighted its effectiveness and safety, reinforcing its credibility among healthcare providers. These findings demonstrate Xarelto’s ability to reduce the risk of serious limb and cardiovascular complications, further strengthening its position as a trusted anticoagulation option.
In the first quarter of 2023, Xarelto saw revenue growth in Europe, helping to offset the impact of increasing generic competition in Canada. However, in the US, where it is marketed by a Johnson & Johnson subsidiary, revenues declined due to mounting competition from generic alternatives.
Despite these challenges, Xarelto remained Bayer’s top-performing pharmaceutical product in 2023. The company’s focus on strategic growth and portfolio management has helped sustain Xarelto’s strong market presence.
Although Xarelto faces pricing pressures and competition, particularly in China and the UK, it demonstrated resilience in Bayer’s portfolio throughout 2023. The first quarter of 2024 reported a 1.7% increase in sales, emphasizing the drug’s continued demand and market significance despite competitive challenges.
In the past, Xarelto encountered legal scrutiny over allegations of severe bleeding risks, leading to a $775 million settlement by Bayer and Johnson & Johnson to resolve most of the related lawsuits without admitting liability. However, the settlement has not diminished healthcare providers’ trust in the drug, which remains widely prescribed due to its proven clinical benefits and safety when used appropriately.
Bayer also faced legal setbacks when the UK High Court invalidated the dosage patent for rivaroxaban, Xarelto’s active ingredient, citing a lack of innovative advancement. This ruling opened the door for generic manufacturers to enter the market, posing a potential threat to Bayer’s market exclusivity. These legal challenges underscore the competitive landscape Bayer navigates as it seeks to maintain Xarelto’s market share amid growing generic competition.
3. Entresto (Sacubitril/Valsartan)
Entresto 2023 sales: $6.04 billion
Company/developer: Novartis
Date of first FDA approval: July 7, 2015
FDA-approved indications: Entresto is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. It is also indicated for the treatment of symptomatic heart failure with systemic left ventricular systolic dysfunction in pediatric patients aged one year and older.
Price of Entresto: The list price of Entresto is $705.21 a month
Why it sold so well: Entresto was the first medication of its class to receive approval for the treatment of chronic heart failure with reduced ejection fraction. Since its introduction, it has become a cornerstone therapy for heart failure patients, particularly after receiving a Class 1 recommendation from the American College of Cardiology (ACC) and the American Heart Association (AHA) in 2016.
Thanks to its proven effectiveness and the absence of generic alternatives, Entresto has consistently delivered strong revenue growth for Novartis. The drug remains protected by patents until 2025. However, the recent FDA approval of generic versions set to enter the market once the patent expires is expected to impact Entresto’s future revenue potential.
4. Opsumit (Macitentan)
Opsumit 2023 sales: $1.97 billion
Company/developer: Actelion Pharmaceuticals US, Inc.
Date of first FDA approval: October 18, 2013
FDA-approved indications: Opsumit is indicated for treating pulmonary arterial hypertension (PAH). It is used to reduce the risks of disease progression and hospitalization related to PAH.
Price of Opsumit: Opsumit is available in 10 mg film-coated tablets, priced at around $128.33 per tablet.
Why it sold so well: Opsumit generated $1.97 billion in global sales in 2023, representing a 10.6% increase from 2022. In clinical studies conducted across 151 centers in 40 countries, Opsumit 10 mg demonstrated a 45% reduction in the first occurrence of morbidity or mortality events compared to placebo. Additionally, the study showed that patients treated with Opsumit experienced a 50% reduction in PAH-related hospitalizations and deaths compared to those receiving a placebo.
5. Repatha (Evolocumab)
Repatha 2023 sales: $1.64 billion
Company/developer: Amgen
Date of first FDA approval: August 27, 2015
FDA-approved indications: Repatha is indicated for reducing the risk of myocardial infarction, stroke and coronary revascularization in adults with established cardiovascular disease. It is also used as an adjunct to diet, alone or with other lipid-lowering therapies (such as statins or ezetimibe), to treat adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), by lowering low-density lipoprotein cholesterol (LDL-C). Additionally, Repatha is indicated as an adjunct to diet and other LDL-C-lowering therapies (such as statins, ezetimibe or LDL apheresis) for patients with homozygous familial hypercholesterolemia (HoFH) who need further LDL-C reduction.
Price of Repatha: The list price of Repatha is $561.49 per month.
Why it sold so well: Repatha sales rose 25% year-over-year in the fourth quarter of 2023 ($417 million), fueled by a 35% increase in volume. For the full year of 2023, sales grew by 26%, driven by a 37% volume increase.
Repatha continues to lead the global PCSK9 segment, with over 2.5 million patients treated since its launch. In Phase III trials, adding Repatha to background lipid-lowering therapy, including statins, led to significant reductions in LDL-C levels and improved other lipid parameters. In patients with clinical atherosclerotic cardiovascular disease (ASCVD) or HeFH, Repatha reduced LDL-C by approximately 54% to 77% compared with placebo.
6. Uptravi (Selexipag)
Uptravi 2023 sales: $1.58 billion
Company/developer: Actelion Pharmaceuticals US, Inc. and Johnson & Johnson
Date of first FDA approval: December 21, 2015
FDA-approved indications: Uptravi is indicated for treating PAH to delay disease progression and reduce the risk of hospitalization.
Price of Uptravi: The average annual cost per patient for Uptravi in the US ranges from $160,000 to $170,000.
Why it sold so well: Uptravi recorded $1,582 million in sales in 2023, marking a 19.7% increase from the previous year. The FDA approved Uptravi based on evidence from a Phase III clinical trial involving 1,156 patients with PAH, who were evaluated for up to 4.2 years. The trial found that the primary composite endpoint was reduced by 40% with selexipag compared to placebo.
In 2021, Uptravi received FDA approval for intravenous (IV) use in patients with PAH. The study that led to the new approval demonstrated that switching from Uptravi tablets to Uptravi IV was well tolerated, with no unexpected safety concerns.
7. Lipitor (Atorvastatin)
Lipitor 2023 sales: $1.56 billion
Company/developer: Warner-Lambert, later acquired by Pfizer
Date of first FDA approval: December 1996
FDA-approved indications: Lipitor is specifically indicated for the prevention of cardiovascular disease, including myocardial infarction and stroke, as well as for the treatment of primary hypercholesterolemia (both heterozygous familial and nonfamilial) and mixed dyslipidemia.
Price of Lipitor: The cost for a 90-tablet supply of Lipitor 10 mg oral tablets is approximately $1,263.
Why it sold so well: In 2019, Pfizer’s Lipitor generated nearly $2 billion in revenue. Even in 2011, when it lost patent protection, Lipitor still generated around $10 billion in revenue. The initial Phase III clinical trial involved 10,305 patients aged 40 to 80 years. The study demonstrated that the drug significantly reduced fatal coronary heart disease, lowering non-fatal myocardial infarction events to 60 compared to 108 in the placebo group. It also decreased the need for revascularization procedures by 42%. Additionally, another clinical study showed the drug reduced the rate of cardiovascular events to 83 compared to 127 in the placebo group, with a 48% reduction in stroke risk and a 42% reduction in myocardial infarction risk.
8. Activase (Alteplase)
Activase 2023 sales: $1.36 billion
Company/developer: Genentech
Date of first FDA approval: November 1987
FDA-approved indications: Activase is indicated for the treatment of acute ischemic stroke (AIS), acute myocardial infarction (AMI) to reduce mortality and the risk of heart failure and for the lysis of acute massive PE.
Price of Activase: The cost of a single 50 mg vial of Activase (intravenous powder for injection) is approximately $4,603.
Why it sold so well: According to a National Institute of Neurological Disorders and Stroke (NINDS) trial, Activase has been shown to reduce disability at 90 days in patients treated within 3 hours of symptom onset. Those receiving Activase within this timeframe experienced less disability and were at least 33% more likely to achieve minimal or no disability at 90 days compared to patients who received a placebo.
9. Adempas (Riociguat)
Adempas 2023 sales: $1.34 billion
Company/developer: Bayer
Date of first FDA approval: October 8, 2013
FDA-approved indications: Adempas is approved for treating adults with persistent or recurrent chronic thromboembolic pulmonary hypertension (CTEPH) after surgical treatment or in cases of inoperable CTEPH, aiming to improve exercise capacity. It is also indicated for PAH to enhance exercise capacity and delay clinical worsening.
Price of Adempas: The manufacturer has listed the price of $42.75 per tablet for all dosage strengths. This equates to a daily cost of $128.25 per patient, or approximately $46,811 annually per patient.
Why it sold so well: Adempas is the first FDA-approved drug in the class of soluble guanylate cyclase (sGC) stimulators. It works by helping arteries relax, which increases blood flow and reduces blood pressure. In a 12-week, Phase III, double-blind clinical trial involving 443 patients with symptomatic PAH, the average 6-minute walk distance increased by 30 meters from baseline for those receiving the maximum 2.5 mg dose of Adempas, compared to a 6-meter decrease in the placebo group. This 36-meter difference between the two groups was statistically significant. Additionally, Adempas showed significant improvements in secondary endpoints, including pulmonary vascular resistance and NT-proBNP levels.
10. Brilinta (Ticagrelor)
Brilinta 2023 sales: $1.32 billion
Company/developer: AstraZeneca
Date of first FDA approval: July 20, 2011
FDA-approved indications: Brilinta is prescribed to lower the risk of cardiovascular death, heart attack and stroke in individuals with acute coronary syndrome (ACS) or those who have previously experienced a myocardial infarction.
Price of Brilinta: Brilinta is around $494 for a supply of 60 tablets.
Why it sold so well: Brilinta remains one of the top-selling drugs in AstraZeneca’s portfolio. The Phase III trial that led to the approval involved 18,624 patients across more than 43 countries and demonstrated that the drug significantly reduced the number of cardiovascular deaths. The study showed that treatment with Brilinta resulted in a greater reduction in the primary endpoint — a composite of cardiovascular death, myocardial infarction or stroke — compared to clopidogrel. Specifically, Brilinta reduced the incidence to 9.8% versus 11.7% for clopidogrel at 12 months, reflecting a 1.9% absolute risk reduction (ARR) and a 16% relative risk reduction (RRR).











Join or login to leave a comment
JOIN LOGIN