Recent years have seen significant advancements and shifts in the therapeutic approaches to one of the world’s leading health concerns: cardiovascular diseases. As we delve into the top 15 cardiovascular disease drugs in 2023, guided by comprehensive 2022 sales data, we gain valuable insights into the market trends, drug efficacy and the evolving needs of patients worldwide.
For healthcare professionals and stakeholders in the life sciences sector, understanding the top-performing cardiovascular drugs is not just about recognizing their financial success. It’s about comprehending the clinical value they bring to patients’ lives, the research and development strides behind their success and their role in shaping future therapeutic pathways.
This analysis not only highlights the leading pharmaceuticals that have carved a niche in cardiovascular disease treatment but also reflects the broader industry movements towards innovative, more effective and patient-centric solutions. From long-standing treatments to novel therapies, the list encapsulates a spectrum of drugs that have proven their mettle in addressing various facets of cardiovascular health, including hypertension, heart failure and thrombotic events, among others.
Read on to learn more about the top 15 cardiovascular disease drugs in 2023, based on 2022 sales statistics.
XTALKS WEBINAR: The MedTech Horizon: Exploring the Frontier of Cardiovascular Device Development in Heart Failure
Live and On-Demand: Thursday, March 14, 2024, at 10am EDT (3pm CET/EU-Central)
Register for this free webinar to learn about the innovation in cardiovascular device development and how it strives to enhance patient outcomes and quality of life.
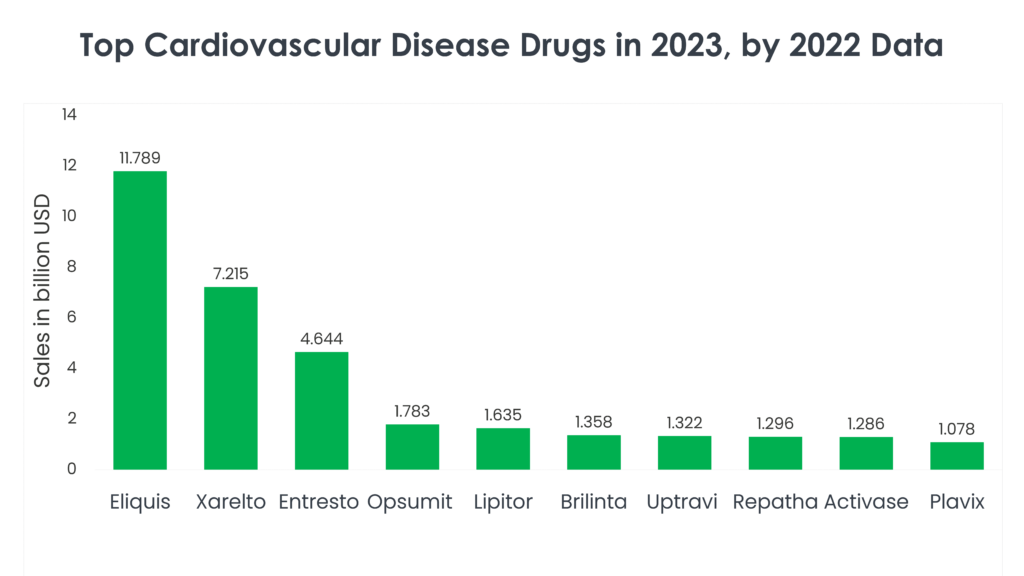
1. Eliquis (Apixaban)
Eliquis sales in 2022: $11.789 billion
Company/Developer: Bristol Myers Squibb (BMS) and Pfizer
Date of first FDA approval: December 28, 2012
Indications Eliquis is FDA-approved for: Eliquis is primarily prescribed to prevent blood clots in individuals with certain medical conditions. This includes reducing the risk of stroke and systemic embolism in non-valvular atrial fibrillation; treating and preventing deep vein thrombosis (DVT) and pulmonary embolism (PE) in patients who have recently undergone hip or knee replacement surgery; and reducing the risk of recurrent DVT and PE after initial treatment. The blood thinner blocks the action of factor Xa, an enzyme involved in the blood clotting cascade, preventing the formation of blood clots that can lead to stroke or other serious medical conditions.
Price of Eliquis: Approximately $635 for a supply of 60 oral 2.5 mg tablets
Why it did so well: Eliquis continues to do extremely well for joint developers BMS and Pfizer, continuing its reign as the top-selling cardiac drug globally. It was also the sixth best-selling drug in the world and BMS’ second best-selling drug in 2022. It has consistently been a top earner for the company for the past couple of years along with oncology drugs Revlimid (lenalidomide) and Opdivo (nivolumab).
Eliquis’ growing success in recent years owes largely to gains in the non-valvular atrial fibrillation market. Along with Janssen’s Xarelto (rivaroxaban), it successfully grabbed a major chunk of the blood thinner market share from warfarin due to several advantages over the legacy drug. Eliquis has been shown to be at least as, or more effective, and safer than warfarin. Additionally, unlike warfarin, it does not require regular monitoring such as the need for routine blood tests. However, sales of Eliquis fell from $16.73 billion in 2021 to $11.79 billion in 2022, which could be attributed to declining sales in certain emerging markets, according to Pfizer’s full-year 2022 results.
2. Xarelto (Rivaroxaban)
Xarelto sales in 2022: $7.215 billion
Company/Developer: Janssen, Pharmaceutical Companies of Johnson & Johnson; Bayer HealthCare AG
Date of first FDA approval: July 1, 2011
Indications Xarelto is FDA-approved for: Xarelto is used in the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation; to treat and reduce the risk of DVT and PE; and in the prevention of blood clots in patients who have undergone hip or knee replacement surgery.
Price of Xarelto: Around $580.71 for a supply of 30 oral tablets (20 mg)
Why it did so well: Xarelto, like close rival Eliquis, is a Xa clotting factor inhibitor that has dominated the blood thinner market with Eliquis for the past several years. Since 2021, Janssen and Bayer have filed patent infringement lawsuits in the US to block generic competition. The class of Xa inhibitors has overtaken the likes of warfarin and Lipitor (atorvastatin) in the oral blood thinner market owing to good efficacy and safety profiles. Marketing in both cardiac and hematological indications has contributed to its success, which continues to grow in emerging markets globally.
3. Entresto (Sacubitril/Valsartan)
Entresto sales in 2022: $4.644 billion
Company/Developer: Novartis
Date of first FDA approval: July 7, 2015
Indications Entresto is FDA-approved for: Reduce risk of cardiovascular death and hospitalization in adults with chronic heart failure; symptomatic heart failure with systemic left ventricular systolic dysfunction in pediatric patients aged one year and older.
Price of Entresto: About $544.75 per month for 60 tablets
Why it did so well: Since its launch in 2015, Entresto has been a growing sales driver for Novartis. In 2021, Entresto’s global sales totaled $3.55 billion, and in 2022, they almost reached $5 billion, the figure Novartis had projected Entresto to hit yearly back in 2018. The company has been getting closer to this goal, particularly after winning expanded FDA approval in both reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) heart failure in 2021. In the same year, the oral, first-in-class angiotensin receptor neprilysin inhibitor also received approval for hypertension in China; it is approved for hypertension in Japan as well. In its 2022 annual report, Novartis said the company delivered on its financials last year owing largely to “continued strong demand” for Entresto.
4. Opsumit (Macitentan)
Opsumit sales in 2022: $1.783 billion
Company/Developer: Janssen, Pharmaceutical Companies of Johnson & Johnson
Date of first FDA approval: October 18, 2013
Indications Opsumit is FDA-approved for: Pulmonary arterial hypertension (PAH)
Price of Opsumit: Around $6,369.43 for 15 oral 10 mg tablets
Why it did so well: In 2022, Opsumit, an endothelin receptor antagonist used to treat PAH, high blood pressure in the arteries of the lungs, continued to lead in the space with $1.783 billion in sales in 2022, up 2.6 percent year over year at constant currencies. In its 2022 annual report, Johnson & Johnson said while pulmonary hypertension products sales were down one percent as compared to the previous year (totalling $3.4 billion), operational sales growth of Opsumit and its other PAH drug Uptravi (seleipag) continued due to gains in shares, while “market growth was offset by COVID-19 related impacts and continued declines in ‘Other Pulmonary Hypertension.’” The company is also battling generic makers in court from entering the market before Opsumit’s patent expires in 2025.
5. Lipitor (Atorvastatin)
Lipitor sales in 2022: $1.635 billion
Company/Developer: Viatris; initially Pfizer
Date of first FDA approval: January 1997
Indications Lipitor is FDA-approved for: Used to lower total cholesterol, low-density lipoprotein (LDL), triglycerides and to elevate high-density lipoprotein (HDL) cholesterol. It is used to reduce the risk of heart attack and stroke and to decrease the chance of heart surgery in people with heart disease or at risk of developing heart disease.
Price of Lipitor: About $1,178.89 for 90 oral 10 mg tablets
Why it did so well: Once upon a time, since 1997 to be precise, Lipitor was the best-selling drug in the world until its patent expired in 2011. Over its nearly 15-year run under patent protection, it amassed a total of $125 billion, becoming one of the best-selling drugs of all time. Lifetime sales of the drug total over $164 billion to date. The blockbuster anti-cholesterol drug’s profile was further boosted during its epic run after trials showed the effectiveness of statins in reducing the risk of heart attack.
Despite new players like Eliquis and Xarelto having grabbed much of the blood thinner market share since their launches in 2012 and after Lipitor’s patent expiration, Lipitor has continued to hold ground thanks to a mammoth legacy and because statins remain popular anti-cholesterol meds. Sales of Lipitor remained relatively steady this year compared to last year, reaching $1.63 billion in 2022 compared to $1.66 billion in 2021. Viatris was formed in 2020 through the merger of Mylan and Uphohn, a legacy division of Pfizer. In the merger, Lipitor was transferred to Viatris.
6. Brilinta (Ticagrelor)
Brilinta sales in 2022: $1.358 billion
Company/Developer: AstraZeneca
Date of first FDA approval: July 20, 2011
Indications Brilinta is FDA-approved for: Used to decrease risk of death, heart attack and stroke in people with acute coronary syndrome (ACS) or a history of a heart attack; decrease risk of a first heart attack or stroke in people with coronary artery disease (CAD) who are at high risk for having a heart attack or stroke; decrease risk of stroke in people having acute ischemic stroke or temporary stroke-like symptoms, known as mini-stroke or transient ischemic attack.
Brilinta Price: About $485 for a supply of 60 oral 60 mg tablets
Why it did so well: Sales of AstraZeneca’s oral antiplatelet Brilinta were down eight percent compared to the previous year ($1.47 billion in 2021). In its 2022 annual report, AstraZeneca said it is expanding Brilinta into new patient populations and emerging markets (excluding China).
AstraZeneca has invested over $5 billion into the anti-clotting drug, primarily on expensive clinical studies. However, it may not be able to recover those costs with sales failing to hit the mark. Sales are expected to take a further hit as generics are set to enter the market this year. The risk of bleeding associated with Brilinta has been an ongoing concern, but PhaseBio may have an antidote with bentracimab. Recent trial data shows bentracimab can rapidly reverse the blood-thinning and antiplatelet effects of Brilinta in as little as five minutes.
7. Uptravi (Selexipag)
Uptravi sales in 2022: $1.322 billion
Company/Developer: Janssen, Pharmaceutical Companies of Johnson & Johnson
Date of first FDA approval: December 21, 2015
Indications Uptravi is FDA-approved for: PAH
Price of Uptravi: 200 mcg is around $15,526 for a supply of 60 tablets; single 1,800 mcg intravenous injection is around $410.90
Why it did so well: Sales of Uptravi were up from around $1.24 billion in 2021 to $1.32 billion in 2022. The PAH duo of Uptravi and Opsumit remains a strong financial driver for Janssen due to good market growth despite the recent impacts of COVID-19, according to Janssen. The robust performance of the selective prostacyclin receptor (IP) agonist and Opsumit held almost half of the PAH market share in 2022, which was worth about $7.3 billion and is expected to hit $12.2 billion in 2032.
8. Repatha (Evolocumab)
Repatha 2022 sales: $1.296 billion
Company/Developer: Amgen Inc.
Date of first FDA approval: August 27, 2015
Indications Repatha is FDA-approved for: To reduce the risk of myocardial infarction, stroke and coronary revascularization in adults with established cardiovascular disease; as an adjunct to diet, alone or in combination with other lipid-lowering therapies (e.g., statins, ezetimibe), for treatment of adults with primary hyperlipidemia (including heterozygous familial hypercholesterolemia [HeFH]) to reduce low-density lipoprotein cholesterol (LDL-C); as an adjunct to diet and other LDL-lowering therapies (e.g., statins, ezetimibe, LDL apheresis) in patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C.
Price of Repatha: The list price of Repatha is $561.49 per month.
Why it did so well: Repatha functions as a PCSK9 inhibitor, designed to lower LDL-C levels in individuals with primary hyperlipidemia and HoFH. Additionally, it aims to diminish the likelihood of cardiovascular events in adults with confirmed cardiovascular disease. With no reported drug interactions, its primary side effect is commonly nasopharyngitis and upper respiratory tract infections. Administered through subcutaneous injection, it offers flexible options, including a prefilled syringe, Sureclick autoinjector, or Pushtronex system, typically administered every two to four weeks for self-administration.
The clinical benefits and safety of Repatha have been studied for 12 years in 50 clinical trials with over 47,000 patients. This vast body of evidence demonstrates that Repatha works rapidly. Moreover, Repatha is approved in more than 75 countries, including the US, Japan, Canada and all 28 countries that are members of the European Union (EU). As per the World Health Organization (WHO), cardiovascular diseases are the leading cause of death globally, taking an estimated 17.9 million lives each year. The sale of Repatha is mainly driven by volume growth which was impacted by the inclusion of Repatha on China’s National Reimbursement Drug List as of January 1, 2022. Repatha remains the global proprotein convertase subtilisin/kexin type 9 (PCSK9) segment leader, with over 1.5 million patients treated since launch.
9. Activase (Alteplase/Tenecteplase)
Activase 2022 sales: $1.286 billion
Company/Developer: Genentech Inc. (a member of the Roche Group)
Date of first FDA approval: November 13, 1987
Indications Activase is FDA-approved for: Activase is a tissue plasminogen activator (tPA) indicated for the treatment of acute ischemic stroke; acute myocardial infarction to reduce mortality and incidence of heart failure; lysis of acute massive PE.
Price of Activase: The cost for Activase intravenous powder for injection 50 mg is around $4,643 for a supply of one powder for injection.
Why it did so well: Activase was one of the first thrombolytic medications approved for acute myocardial infarctions and has consistently performed well. The key market drivers include the increasing geriatric population and the rise in the prevalence of hypertension, kidney diseases, strokes and other cardiovascular diseases. Stroke and heart disease are major global health concerns and leading causes of morbidity and mortality. The rising incidence and prevalence of these conditions drive the demand for alteplase, an essential thrombolytic treatment for acute ischemic stroke and myocardial infarction. Roche reported a 15 percent increase in sales in their half-yearly report for 2023 compared with 2022 for the same period, which is a strong indicator of consistent market growth.
10. Plavix (Clopidogrel)
Plavix 2022 sales: $1.078 billion
Company/Developer: BMS/Sanofi Pharmaceuticals partnership
Date of first FDA approval: November 17, 1997
Indications Plavix is FDA-approved for: Plavix is a P2Y12 platelet inhibitor indicated for: ACS; recent myocardial infarction, recent stroke, or established peripheral arterial disease.
Price of Plavix: The cost of Plavix oral tablet 75 mg is around $118 for a supply of 30 tablets.
Why it did so well: Plavix, which was once the gold standard blood thinner, has been losing revenue owing to cheaper generic versions being available in the market after its patent expiry for over a decade now. Despite facing strong competition, it is generating stable revenue. During the first six months of 2023, net sales of Plavix were reported to be $501 million as sales growth in the Rest of the World region offset lower sales in the US and Europe. The market growth can be attributed to the rise in the geriatric population and the rise in cardiovascular diseases.
11. Jynarque (Tolvaptan)
Jynarque 2022 sales: $1.053 billion
Company/Developer: Otsuka Pharmaceutical Co.
Date of first FDA approval: Tolvaptan was first approved by the FDA in 2009 as Samsca for the treatment of clinically significant hypervolemic and euvolemic hyponatremia. This was followed by another approval in 2018 to slow kidney function decline.
Indications Jynarque is FDA-approved for: Slow kidney function decline in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease (ADPKD).
Price of Jynarque: The cost for Jynarque oral tablet (45 mg-15 mg) is around $20,227 for a supply of 56 tablets.
Why it did so well: ADPKD affects one in every 400 to 1,000 people and is the most common kidney disorder passed down through family members. This debilitating disease has a 50 percent chance of being passed down from parent to child. About 140,000 people in the US are diagnosed with ADPKD, which is characterized by the development of kidney cysts.
In May 2018, Jynarque was launched in the US as the first and only approved treatment for ADPKD. Efforts to raise disease awareness and make clinical trial data available have helped spread recognition of both the disease and the drug, resulting in a steady increase in prescriptions. In Europe also, Jinarc is sold in approximately 20 countries and the number of patients receiving treatment through the drug is on the increase. It is patent protected until 2026; therefore, the revenue is safe from biosimilar competition for now.
12. Crestor (Rosuvastatin)
Crestor 2022 sales: $1.048 billion
Company/Developer: AstraZeneca
Date of first FDA approval: August 13, 2003
Indications Crestor is FDA-approved for: To reduce the risk of stroke, heart attack and the need for procedures to improve blood flow to the heart called arterial revascularization in adults who do not have known heart disease but do have certain additional risk factors.
Crestor is used along with diet to: lower the level of LDL-C or “bad” cholesterol in adults with primary hyperlipidemia; slow the buildup of fatty deposits (plaque) in the walls of blood vessels; treat adults and children eight years of age and older with high blood cholesterol due to HeFH; along with other cholesterol-lowering treatments or alone if such treatments are unavailable in adults and children seven years of age and older with HoFH; treat adults with a type of high cholesterol called primary dysbetalipoproteinemia (type III hyperlipoproteinemia); lower the level of fat in the blood (triglycerides) in adults with hypertriglyceridemia.
Price of Crestor: The cost of Crestor oral tablet 5 mg is around $172 for a supply of 30 tablets.
Why it did so well: Crestor has been the best-selling anti-cholesterol pill by AstraZeneca which was mainly due to the fact that it was highly effective in reducing LDL-C by up to 55 percent (at 20-mg dose versus seven percent with placebo). After its patent protection expired in 2016, its sales have been declining, but it remains one of the major revenue generators for AstraZeneca till now. Almost two in five adults in the US have high cholesterol (total blood cholesterol ≥ 200 mg/dL). The rising incidence of high cholesterol is one of the top drivers for Crestor sales globally.
13. Tyvaso (Treprostinil)
Tyvaso 2022 sales: $873 million
Company/Developer: United Therapeutics Corp
Date of first FDA approval: July 30, 2009
Indications Tyvaso is FDA-approved for: Tyvaso is a prostacyclin mimetic indicated for the treatment of PAH to improve exercise ability; and pulmonary hypertension associated with interstitial lung disease (PH-ILD) to improve exercise ability.
Price of Tyvaso: The cost for Tyvaso inhalation solution (0.6 mg/mL) is around $3,154 for a supply of 11.6 mL.
Why it did so well: Tyvaso is the top revenue generator for United Therapeutics Corp and net product sales of treprostinil-based products (Tyvaso DPI, nebulized Tyvaso, Remodulin and Orenitram) grew by $89.7 million, or 20 percent, for the third quarter of 2023, as compared to the third quarter of 2022. The growth in total Tyvaso revenues resulted primarily from an increase in total quantities sold driven by the commercial launch of Tyvaso DPI in June 2022 and continued growth in use by patients with PH-ILD. Moreover, Tyvaso is the first and only approved therapy in the US for patients with PH-ILD, a serious, life-threatening disease.
14. Adalat (Nifedipine)
Adalat 2022 sales: $872 million
Company/Developer: Bayer HealthCare Pharmaceuticals Inc.
Date of first FDA approval: December 31, 1981
Indications nifedipine is FDA-approved for: Chronic stable angina, vasospastic angina and hypertension
Price of Adalat: The price of Adalat CC oral tablet (extended-release 30 mg) was reported to be around $165 for a supply of 100 tablets.
Why it did so well: The Adalat CC brand name has been discontinued in the US, but the generic form of the drug is available in the US. Overall, the nifedipine medication market is poised to witness significant growth in the coming years due to the growing global burden of cardiovascular diseases and increasing healthcare expenditure.
15. Seloken (Metoprolol)
Seloken 2022 sales: $862 million
Company/Developer: AstraZeneca
Date of first FDA approval: August 7, 1978
Indications metoprolol is FDA-approved for: Metoprolol systemic is used in the treatment of angina; angina pectoris prophylaxis; aortic aneurysm; atrial fibrillation; benign essential tremor; heart attack; heart failure; high blood pressure; left ventricular dysfunction; migraine prevention; mitral valve prolapse; premature ventricular depolarizations; and supraventricular tachycardia.
Price of metoprolol: The price of Metoprolol Tartrate (metoprolol) oral tablet 25 mg is around $10 for a supply of 14 tablets.
Why it did so well: Seloken is not available in the US, but the generic version (metoprolol) is available in the US. A major driving factor for sales of metoprolol is the rising incidence of hypertension and cardiovascular diseases. With an increase in the geriatric population in the US, sales are expected to experience consistent growth.











Join or login to leave a comment
JOIN LOGIN