Radiobiological and Clinical Considerations from CRO and Emerging Biotech
Radiopharmaceuticals are medicinal formulations containing radioisotopes that are used in the field of nuclear medicine primarily for the diagnosis and treatment of various diseases, particularly cancer.
Therapeutic radiopharmaceuticals are particularly used in the treatment of cancer. These include isotopes like iodine-131 for treating thyroid cancer or yttrium-90 for targeted radiotherapy in certain cancers. Therapeutic radiopharmaceuticals deliver beta or alpha radiation, which destroys diseased cells, such as cancer cells. The aim of these compounds is to deliver radiation directly to the tumor cells, minimizing damage to surrounding healthy tissues.
Since Henri Becquerel’s discovery of natural radiation in 1896, Marie Curie’s Nobel Prize-winning work in discovering radium and polonium and William H. Briner, known as the father of radiopharmacy, having paved the way for its practice, the use of radiopharmaceuticals in medicine continues to grow and evolve. In 2023, there have been pivotal trends shaping the landscape and application of alpha emitter therapy. There have been more than $1.7 billion influx into radiopharmaceuticals which underscores the potential and robust growth in this therapeutic area. Over the last year, there have been new acquisitions, launches and deals for novel radiotherapeutics and newly created peptide-radioisotope drug conjugates. Regulatory bodies have demonstrated preparedness for the surge in radiopharmaceuticals as evidenced by inaugural Chemistry, Manufacturing and Controls (CMC) Development and Readiness Pilot (CDRP) programs to expedite commercial manufacturing, US Food and Drug Administration (FDA) approvals and marketing authorizations.
In a recent webinar by Medpace, Jess Guarnaschelli, MD, Medpace Radiation Oncologist, Dr. Jason T. Anderson, PharmD, PhD, Radiation Clinical Pharmacologist, A. Omer Nawaz, PhD, Molecular Imaging Director of an emerging biotech, and Michael Lamba, PhD, DABR, Academic Medical Physicist, explored recent breakthroughs in radiotherapy. The discussion centered on the evolving potential of alpha particle therapy for treating advanced and metastatic tumors.
Alpha particle therapy, also known as alpha radiotherapy or alpha-emitting radionuclide therapy, is a form of targeted treatment that uses molecules labeled with alpha-emitting radionuclides to selectively target and destroy cancer cells.
In 1997, bismuth-213 was the first alpha emitter to be used clinically. In 2013, the approval of Xofigo (radium-223) was a significant milestone for targeted alpha therapy as it was the first-in-class alpha emitter to receive US Food and Drug Administration (FDA) approval and to date, remains the only approved targeted alpha therapy. The approval was a major step forward in the treatment of metastatic castration-resistant prostate cancer, explained Dr. Guarnaschelli in the webinar. She said after the approval of Xofigo, “alpha emitters hit the ground running.” Currently, several alpha particle emitters are being investigated in clinical trials.
Alpha Particle Therapy
While alpha emitter isotope therapy has been around for over a century, there has been renewed interest in the therapeutic potential of alpha emitters to treat advanced cancers. Recent evidence has shown the impressive safety and tumor responses of alpha particle therapy across various solid tumors.
Alpha particles are helium nuclei consisting of two protons and two neutrons. They are highly charged and have a high linear energy transfer (LET), which means they can release a large amount of energy over a short distance. The high LET and short range of alpha particles offer a therapeutic advantage over beta particles, which are used in traditional radiotherapy. Alpha particles cause more localized and intense damage, reducing the risk of harm to adjacent healthy tissues.
When alpha-emitting radionuclides are delivered to cancer cells, the emitted alpha particles cause substantial damage to the DNA of the cells, leading to cell death. Due to their short range, alpha particles can destroy cancer cells while minimizing damage to surrounding healthy tissue.
To specifically target cancer cells, alpha-emitting radionuclides are often attached to molecules that have a high affinity for cancer cells, such as antibodies or peptides (ligands). These ligands can bind to cancer-specific antigens or receptors, delivering the alpha-emitting radionuclides directly to the tumor.
Alpha particle therapy has shown promise in treating several types of cancers, including prostate cancer, neuroendocrine tumors and some types of leukemia and lymphoma. It is particularly useful for treating metastatic disease and tumors that are difficult to target with conventional therapies.
Common radionuclides used in alpha therapy include radium-223, actinium-225 and bismuth-213. Xofigo (radium-223 dichloride), for instance, is used in the treatment of metastatic castration-resistant prostate cancer that has spread to bones.
Alpha Emitter Radioligand Therapy Versus External Beam Radiation Therapy
External beam radiation therapy is generally delivered through a linear accelerator with photons or electrons. External beam radiation, which includes 2D, 3D or image-guided radiation therapy (IGRT), has been around for decades. External beam radiation therapy is administered in gray (Gy) or centigray (cGy) with widely accepted normal tissue tolerances with standard fractionation. There are well-defined dosimetry procedures and standards in place for their use.
Generally, a fractionated treatment regimen is given for which well-defined dosimetric guidelines are in place, explained Dr. Guarnaschelli. The toxicity associated with these radiation modalities is expected within the planned treatment field, and also the planned target volume (PTV) around it, the radiobiology and modes of DNA damage are well studied and understood. However, for alpha emitter therapies, practices are not yet standardized for normal tissue tolerances and dosimetry.
Radiopharmaceutical alpha therapy involves a relatively lower whole-body absorbed dose than external beam radiation therapy. Thus, it is possible that radiopharmaceuticals with alpha therapy can increase the therapeutic index, noted Dr. Guarnaschelli.
Other differences between the two radiation modalities include the fact that alpha emitter therapy is delivered intravenously. There is a small but real risk of extravasation as well as a known systemic effect with intravenous administration. Alpha therapies are most often implemented in metastatic or palliative settings and the authorized user or physician would prescribe an activity, explained Dr. Guarnaschelli.
Advantages of Alpha Emitter Therapy
Over the past two decades, the radiological and chemical properties of alpha emitting isotopes have regained prominence in medical physics. As cancer treatments, alpha emitter radiopharmaceuticals hold great potential in delivering a highly cytotoxic dose to cancer cells while lessening damage to surrounding normal tissue due to the short range and high LET of alpha particles.
Additionally, alpha therapy could help circumvent radiobiologic adaptive resistance and cell cycle progression, which are hurdles associated with conventional radiation therapy.
It is also important to note that there are key differences between the radiobiological nature of beta emitters and alpha emitters. The alpha particle in alpha emitter therapy is 8,000 times heavier than beta particles. Other differences include:
- Ionizing Power: Moderate for beta emitters, higher for alpha emitters
- Range of Travel: Long for beta emitters, shorter for alpha emitters
- LET: Low for beta emitters, higher for alpha emitters
- Type of DNA Damage: Beta emitters cause single-strand breaks while alpha emitters cause double-strand breaks
Alpha Radionuclide Therapy: Clinical Considerations
Given that tumors and micrometastases consist of heterogeneous cell populations, there are many complex dynamics on a cellular level that must be considered when delivering alpha therapy tumors.
Importantly, Dr. Guarnaschelli explained that it isn’t only the cell nucleus that plays a role in the cancer cell outcome as after irradiation, many other complex cell mechanisms are impacted and involved. For example, non-target effects such as the release of damaged DNA into the cytoplasm of irradiated cells can lead to the activation of immune cells. Other non-target effects include the communication of irradiated cells with neighboring cells, which is called the bystander effect, and at longer distances, irradiated cells can even activate immune cells. These can manifest as immediate or late effects, which can ultimately impact the efficacy of treatment.
There are also many clinical considerations when administering alpha emitters. In terms of radiation acute side effects, these treatments may include nausea, vomiting, xerostomia (dry mouth), alopecia, radiation sickness and bone marrow suppression. Late sequela could include pneumonitis, lung fibrosis, fertility disorders and secondary malignancies.
Radiobiological Considerations of Alpha Emitters: No Two Are the Same
While Xofigo (Ra-223) remains the only FDA-approved alpha emitter, several others are being investigated in both clinical trials and preclinical studies. Some of the ones at the forefront include lead-212 (Pb-212) and actinium-255 (Ac-225).
With respect to the decay cascades of alpha emitters, not all alpha emitters are the same. For example, lead and actinium emit different amounts of alpha particles and at different times. They also all have different energies. In addition, the cascades can also contain beta emissions. Hence no two alpha emitters, despite emitting the same alpha particle, are the same.
This makes the radiobiological considerations of alpha emitters important, which include dose, LET and other considerations such as tumor control probability (TCP) and normal tissue complication probability (NTCP), which are parameters used to calculate the percentage of tumor killing and effects on normal tissue damage, respectively, as well as modeling equivalent dose in two Gy fractions (EQD2).
At the crux of radiobiological considerations are the 5Rs of radiobiology (listed below), which describe the biological response of cells and tissues to radiation. They are fundamental in understanding how radiation therapy works in treating cancer and in predicting and managing the side effects of treatments. They apply to all radiotherapy modalities for the most part, including alpha emitters to some extent.
The 5 Rs of Radiobiology:
Repair: This is the consideration of what DNA repair mechanisms are activated to repair DNA in both tumor and healthy cells. The efficiency of repair processes can affect how cells survive after radiation therapy. This is crucial as the ability of cancer cells to repair DNA damage can influence their resistance to radiation treatment.
Repopulation: Both healthy tissue and cancer cells can survive and proliferate after radiation therapy. In normal tissues, this helps in healing, but in tumors, this can lead to regrowth. The rate of repopulation is a critical factor in the outcome of fractionated radiation therapy, where the treatment is given in several small doses.
Reoxygenation: The amount of oxygen inside tissue impacts tumoricidal doses with respect to LET and single-stranded breaks. Tumors often contain areas with low oxygen levels (hypoxia), which are more resistant to radiation. After initial radiation treatment, these hypoxic areas can become reoxygenated, making them more sensitive to subsequent radiation treatments. Reoxygenation improves the efficacy of radiation therapy over multiple sessions.
Redistribution: Cells have differing sensitivities to radiation during different stages of the cell cycle. Radiation therapy can cause surviving cells to redistribute into more sensitive phases of the cell cycle, making them more vulnerable to subsequent radiation treatments.
Radiosensitivity: The inherent sensitivity of different cell types to radiation relates to the histology of the tumor, i.e., sarcomas are more radioresistant than adenocarcinomas whereas cancers, such as lymphomas are less radiosensitive. The effectiveness of radiation therapy depends partly on the radio sensitivity of the tumor cells compared to that of the surrounding normal cells. Tailoring radiation doses based on the radiosensitivity of the target tissue is important for maximizing treatment efficacy while minimizing damage to normal tissues.
Other Radiobiological Considerations and Overcoming the Five Rs with Alpha Emitters
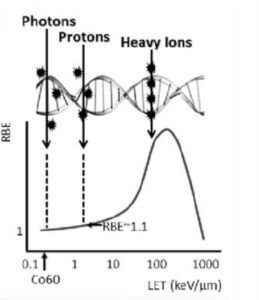
effectiveness (RBE) is maximized
and peaks around 100 keV/µm, which is at
the length and width of a DNA double helix.1
Linear Energy Transfer (LET)
The LET refers to the amount of energy an ionizing particle deposits to the material per unit distance. This is an important parameter because there is a “sweet spot” where the radiobiological effectiveness (RBE) is maximized as demonstrated in Figure 1 in which the curve starts out at one and then peaks around 100 keV/µm. This is because as the particles traverse through tissue, they deposit energy and the sweet spot exists exactly at the length and the width of a DNA double helix, explained Dr. Nawaz. Therefore, if a particle can deposit its energy within the same interval as the length of a double helix, it will result in a double-stranded break.
Oxygen Enhancement Ratio
When cells are treated with traditional radiotherapies like X-rays and beta emitters, they only affect the outer layer of a tumor or tissue as cells in the middle may be hypoxic. The single-stranded breaks that occur in the hypoxic cell will be repaired versus the ones that have a higher amount of oxygen. Therefore, dosing several times to essentially take one layer of a tumor off at a time is usually required versus alpha therapies, which do not depend on oxygen enhancement ratios or some of the other radiobiology considerations. This is because they’re in that sweet spot of LET that they don’t care much about how much oxygen there is and can use dominant double-stranded breaks to maximize cell kill, explained Dr. Nawaz.
While the oxygen enhancement ratio that external beam and beta therapies and every other form of radiotherapy depend on is high at low LETs, a high LET particle does not require high levels of the oxygen enhancement ratio.
“As you increase LET and get to that sweet spot of the DNA double helix, the oxygen enhancement ratio goes down to almost nothing, meaning that it doesn’t really matter how much oxygen you have in the tissue when you have a high LET particle. We may be able to toss out one of the radiobiology considerations,” Dr. Nawaz noted.
Importance of Linear Transfer
Dr. Nawaz explained that proton therapy has become highly sought after just for their ability to increase LET from 0.03 to 0.05. Meanwhile, the LET for alpha particles ranges from 50 to 200, which “demonstrates the power of the alpha particle compared to traditional radiation therapy,” said Dr. Nawaz. With increasing LET, the efficiency of cell killing goes up (Figure 2). For example, if you target ten cells with alpha therapy, ten cells are killed. With X-rays on the other hand, the kill isn’t as efficient, as you may hit ten cells but only kill five, and there will be subsequently more cells.
Alpha emitters are densely ionizing, which means that when they traverse through tissue, they ionize everything along the way until they stop. This is in contrast to photons, protons and beta emitters. And the LET is near perfect as it’s exactly where we need the double-stranded helix to be, which is around two nanometers, he explained.
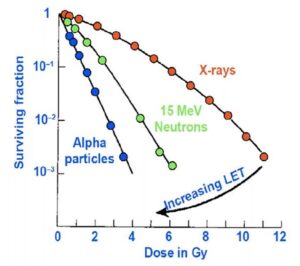
for cell killing with different
radiotherapy modalities. Cell
killing depends on the LET of
the irradiation, with efficiency
increasing with LET.2
Therefore, the five Rs of radiobiology when considering regular radiation therapy such as beta emitters, electrons, photons and protons may not apply to alpha emitters because there’s no sublethal damage and there is primary reliance on double-stranded breaks. Additionally, there’s no cell cycle distribution to consider and hence fractionation can be reconsidered. There’s also no oxygen enhancement ratio that appears to have a major effect, nor repopulation or any dose rate dependency that’s been observed. There are of course differences between different alpha emitters in terms of how they may act.
These physical properties such as high LET, in combination with the radiobiological considerations discussed, are the primary reason for when radiation absorbed dose (in Gray or Joules/kg) is calculated for alpha particles, a multiplication factor of five is recommended when comparing to standard absorbed dose calculations of beta or gamma emitters. This multiplication factor is referred to as the Relative Biological Effectiveness (RBE) and further demonstrates the potency of alpha particles in which 1 Gray of absorbed dose from Alpha particles is equivalent to 5 Gray using conventional beta and gamma particles.
Pharmacokinetic Considerations of Alpha Emitters
The goal of optimal dose selection is to determine a dose that maximizes efficacy while minimizing toxicities. Data is ideally used to drive these decisions, using both pharmacokinetics (PK), which focuses on the change in concentration over time, and pharmacodynamics (PD), which connects a specific biologic effect over time, to derive exposure versus response, explained Dr. Anderson.
This approach is also supported by the FDA’s Project Optimus initiative, which focuses on using exposure response as the rationale for the dose selection rather than just solely utilizing the maximum tolerated dose, said Dr. Anderson.
While both imaging and PK are used to determine exposure for beta emitters, there are imaging challenges for alpha emitters, which leads to a greater reliance on PK as a measure of exposure. With respect to radiation-induced toxicities, adverse events are typically specific to the radiopharmaceutical. Factors that influence toxicities include the radionucleotide, the targeting agent and the predominant pathway for clearance.
Renal Radiation Limits
The renal route is typically the primary clearance pathway for therapeutic radiopharmaceuticals.
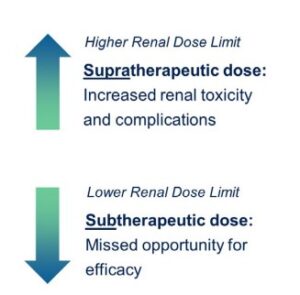
Limits3,4
In the past, limits of 18 to 23 Gy have been set based on renal limits, which were limits determined using external beam radiation. Depending on the presence or absence of risk factors such as hypertension or a geriatric population, a range of 28 to 40 Gy can be used as the guidance for the biological effective dose (BED). Determining an accurate exposure limit is critical to the outcome of a study because if the renal limit is too high, there are increased incidence of renal toxicities. And if the bar is set too low, patients may not reach optimal exposures and may not get the full benefit of the treatment, explained Dr. Anderson.
While the paradigm of the radiobiological considerations of external beam therapies may not be applicable for alpha emitters, the same discussion could be held around renal limits since heterogeneity of dose distribution or differences in energy delivered could play a role in differing effects on the kidneys.
Dr. Anderson said FDA guidance and recommendations in the field include the collection of data early and leveraging this data to drive evidence-based clinical decisions. This could include the use of blood PK to determine whether the correct exposure has been obtained and whether higher exposures will lead to more side effects or efficacy. Urine PK could also be evaluated to determine the total involvement of the kidneys and how much is cleared within the first 24 or 48 hours, for example. As noted earlier, the accumulated dose within the kidneys is also an important consideration during the dose selection process. Since every pharmaceutical is unique, closely monitoring labs such as estimated glomerular filtration rate (eGFR) to detect any changes in renal function with increasing exposures is also critical.
Recent Advances in Radiotherapy
There are many parallels between the historical developments in the well-utilized external beam radiotherapy and developments in radiopharmaceutical therapy, said Dr. Lamba.
There have been a number of advancements common to both external beam and radiopharmaceutical therapy. Some of these include:
Patient Models
There have been significant improvements in patient models. For external beam therapy, the field has moved from simple 2D models to 3D patient-specific anatomy and patient-specific-dose delivery. For radiopharmaceutical therapy, models have also advanced significantly, from simple hermaphrodite wireframe models to anatomically correct models and even patient-specific models, explained Dr. Lamba.
Dose Delivery
There have also been significant improvements in the precision of dose delivery. In external beam radiotherapy, precisely aimed beams of radiation have been developed to deliver highly localized and targeted radiation, such as stereotactic radiosurgery in which the aim is to sterilize the tissue within the targeted region. Similarly, with the advent of targeting molecular pathways with appropriate payloads, radiopharmaceutical therapy can deliver highly localized and precise, biologically aimed radiation.
Particle Types
The types of particles used for therapy have also been augmented, noted Dr. Lamba. In external beam radiotherapy, recent advances have included proton therapy and heavy ion particle therapy. Proton therapy can deliver doses to targets while sparing normal tissue. Similarly, with radiopharmaceutical therapy, the dose deposition kernel from an alpha particle is delivered in a very localized way in which the LET ionizations extend only 50 to 100 microns from the target. In contrast, beta particles with much less densely ionizing radiation extend a lot further, in the order of millimeters to even one centimeter.
Imaging and Dosimetry
There have been improvements in the mechanism of dose delivery as well. In intensity-modulated radiation therapy (IMRT), mathematical optimization of the treatment allows for the delivery of the desired dose to the target tissue while limiting dose to organs at risk. With radiopharmaceutical therapy and the advent of personalized hybrid imaging and treatment planning, quantitative images can be acquired after a test administration and that information can be used to perform physical dosimetry and bioeffect modeling to determine a maximum tolerable dose to an organ at risk, explained Dr. Lamba. These can be used to personalize the efficacy of the treatment.
Dynamic, Personalized Treatment
In external beam therapy, adaptive radiotherapy involves dynamic observation of the therapy through the course of treatment, allowing for altering treatment to changes in patient anatomy and tumor shrinkage. Similarly, for radiopharmaceutical fractionated therapy, with patient-specific voxel dosimetry, patients can be followed through the course of treatment to deliver the appropriate dosage to maximize efficacy on a personalized basis.
The advancements in external beam radiotherapy and radiopharmaceutical have many parallels. However, external beam radiotherapy can be largely controlled by physics of the delivery system. With radiopharmaceutical therapy, in addition to the physics, the biological targeting, uptake, retention and excretion pathways can vary considerably from patient to patient. This biological variability adds complexity, but with that complexity comes the power to choose the targets, the tags and the payloads and select combinations appropriate to the patient to personalize that therapy.
There is also potential for using several radiotherapies in combination therapies. For example, external beam could be used for large tumor burdens, beta particles for targeting tumor stromal tissues and alpha particles for the highly specific tumor cells, explained Dr. Lamba. Radiotherapies can also be combined with other therapies such as chemotherapy, immunotherapy or other targeted therapies. And with further development and experience, radiopharmaceutical therapy will likely be used earlier in the course of treatment, potentially further improving its efficacy.
The field of radiopharmaceuticals is continually evolving with research focused on developing agents that can target diseases more effectively, with lower toxicity and better patient outcomes. Advances in molecular biology and chemistry are aiding in the creation of more specific and effective radiopharmaceuticals.
There are a number of unique and complex radiobiologic considerations for alpha therapy. There are also several challenges, such as the challenge of imaging alpha emitters, which has hindered standardizing dosimetry, leading to the importance of using PK in early-phase trials.
Alpha particle therapy represents a significant advancement in cancer treatment, reflecting the growing trend in oncology towards personalized and precision medicine.
Accelerating Global Radiopharmaceutical Clinical Development
To arrive at success in your radiopharmaceutical development, it takes a team-based approach to drive evidence-based clinical decisions. Our in-house team of radiation oncologists and imaging experts understand the biological, clinical, regulatory, operational and imaging considerations that must be factored in when designing and executing radiopharmaceutical trials. Contact our team of experts today.
To learn more about advancements in the field of alpha emitter therapy and radiopharmaceuticals, watch Medpace’s on-demand webinar.
Full-Service Clinical Development
Medpace is a scientifically-driven, global, full-service clinical contract research organization (CRO) providing Phase I-IV clinical development services to the biotechnology, pharmaceutical and medical device industries. Medpace’s mission is to accelerate the global development of safe and effective medical therapeutics through its high-science and disciplined operating approach that leverages local regulatory and deep therapeutic expertise across all major areas including oncology, cardiology, metabolic disease, endocrinology, central nervous system and anti-viral and anti-infective.
References
- The Green Journal. 2018. Radiotherapy and Oncology.
- Relative Biological Effectiveness (RBE), RBE~3-7 but depends on AD, biological endpoint, tissue type as discussed by Donika Plyku, PhD (FDA)
- Dawson, Laura A., et al. “Radiation-associated kidney injury.” International Journal of Radiation Oncology* Biology* Physics 76.3 (2010): S108-S115.
- Bodei, Lisa, et al. “Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90 Y-DOTATOC and 177 Lu-DOTATATE: the role of associated risk factors.” European journal of nuclear medicine and molecular imaging 35 (2008): 1847-1856.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.





Join or login to leave a comment
JOIN LOGIN