Results of a Phase III clinical trial conducted by AbbVie for its Bcl-2 inhibitor, venetoclax, show that combining it with azacitidine extends overall survival (OS) in patients with untreated acute myeloid leukemia (AML). AbbVie announced the positive results from the first interim of its VIALE-A trial that evaluated the efficacy and safety of the combination therapy, which yielded improvements in the primary endpoints of OS and composite complete remission rate in previously untreated AML patients. This lends hope for an improved treatment strategy for this highly-aggressive and difficult-to-treat cancer.
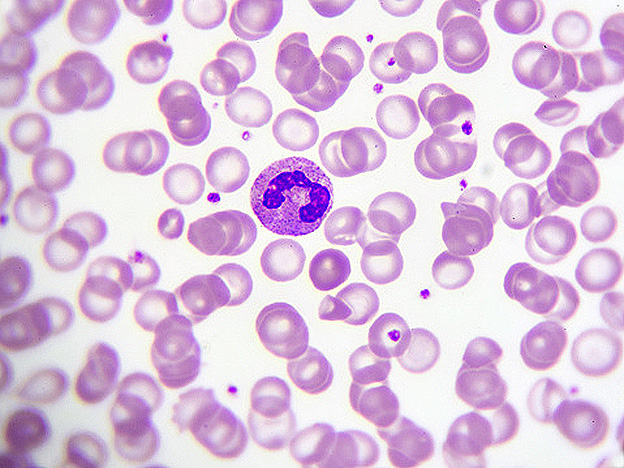
AML is a heterogeneous and acutely fatal malignancy of the bone marrow that arises from acquired mutations in blood cell precursors (i.e. blood stem or progenitor cells) that cause a block in their differentiation and growth into mature blood cells. The accumulation of these aberrant immature cells leads to the leukemia, which involves a ‘bone marrow failure’ state in which there is compromised production of normal blood cells, coupled with the rapid growth of leukemic cells.
Survival outcomes for AML are poor with five-year survival rates being under 30 percent. This reflects the difficulty in treating the cancer, largely owing to its high degree of heterogeneity and aggressiveness. The standard induction chemotherapy treatment regimen for AML is the ‘7+3’ protocol, which involves administration of cytarabine for seven days, followed by daunorubicin for three days – this regimen has remain unchanged for the past several decades, and while patients are able to achieve remission with induction treatment, the majority of them relapse within two years. There is therefore a great need to develop new and effective therapies for the disease.
Venetoclax is a targeted Bcl-2 inhibitor that was jointly developed by AbbVie and Roche under the trade name Venclexta. Venetoclax inhibits the B-cell lymphoma-2 (Bcl-2) protein, which functions to prevent programmed cell death (apoptosis) by maintaining the integrity of the outer mitochondrial membrane in cells; therefore, using it against cancer cells would be an effective way to induce their death.
Venetoclax is currently approved by the US Food and Drug Administration (FDA) for the treatment of adults with chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL). It was also fast-tracked for approval in 2018 for use in AML in combination with azacitidine, decitibine and low-dose cytarabine in patients older than 75, or who have comorbidities that preclude them from receiving intensive induction chemotherapy.
These combinations are part of AbbVie’s three-trial agreement to evaluate the efficacy of these treatments for AML. Earlier in the month, results from the Phase III venetoclax and low-dose cytarabine trial (VIALE-C) were reported, showing that the combination was ineffective in improving OS in AML patients.
The Phase III VIALE-A venetoclax and azacitidine trial involved 443 treatment-naïve (i.e. not previously treated) AML patients, of which 433 were randomized in the double-blind placebo-controlled trial. The efficacy and safety of the venetoclax and azacitidine combination were compared to a placebo and azacitidine combination. Patients were given a daily 400 mg dose of venetoclax along with azacitidine. Results of the study showed statistically significant improvements in OS and composite complete remission, which were the primary endpoints of the study. The safety profile was shown to be consistent with the safety profiles of venetoclax combined with azacitidine as evaluated in previous Phase I/II studies, as well as the known individual safety profiles of the two drugs.
At the recommendation of an independent data monitoring committee (IDMC) and in accordance with a pre-determined interim analysis plan, the positive efficacy results achieved at the first interim analysis will be reported early, along with submission of trial data to the FDA and global health agencies. Results from the interim analysis will be presented at medical/scientific meetings and published in a peer-review journal shortly.
The results from the trial are encouraging and while the combination therapy holds promise, it is important to note that the trial was conducted in largely older patients and/or patients ineligible for standard induction chemotherapy. It will be interesting and important to evaluate the efficacy of the combination in other subsets of AML patients as either a single combinatorial treatment strategy, or as adjuncts to standard induction chemotherapy, if suitable.


Join or login to leave a comment
JOIN LOGIN