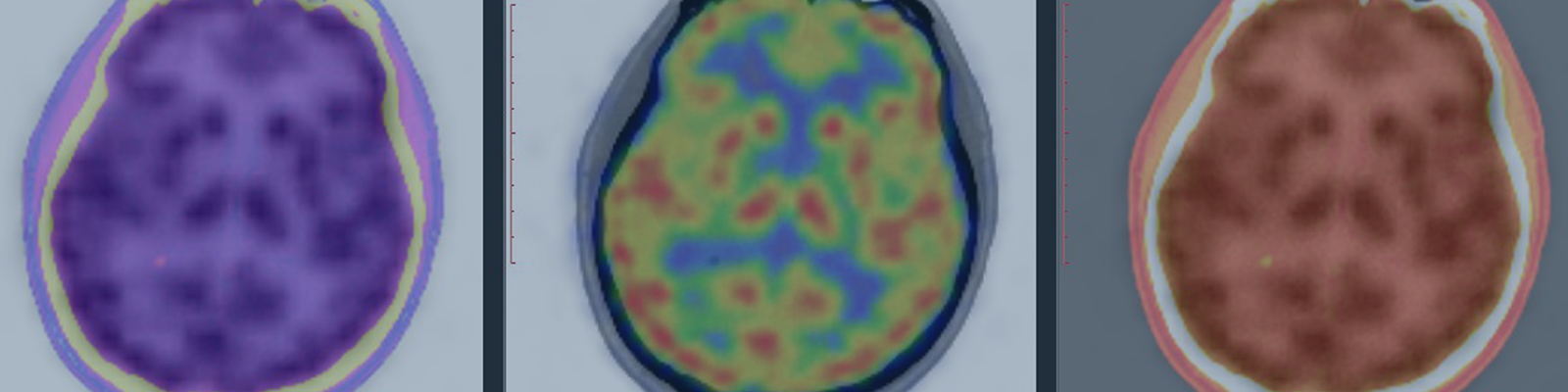
Crohn’s Disease (CD) is an Inflammatory bowel disease affecting the lining of the gut and can occur in different areas of the digestive tract from the mouth to the anus. It is common in CD to have a distribution of random inflamed bowel adjacent to normal gut.
Video endoscopy methods predominate in diagnostic practice but are limited to identifying bowel strictures and the surface of the bowel mucosa. MRI in the abdomen is non-invasive, utilized non-ionizing radiation, and permits varying contrast agents. Magnetic resonance enterography (MREn) can be used as a complement to endoscopy with the advantage of evaluating sub-surface features of CD. Patient preparation and site training are important components of a successful multisite clinical trial, and details will be shared from our experiences on how to manage site for trial success. MREn is commonly scored using the MaRIA calculation, a composite score with important inputs like wall thickness, relative contrast enhancement, edema and ulcerations.
In this scoring system, an expert reader evaluates each of 6 bowel segments to generate a global score, indexed to severity. In a subset of CD patients, MREn methods can be used to assess perianal fistula, and this has greatly expanded from just simple fistula location identification.
Register for this webinar to learn about the image acquisition requirements and various MREn scoring methods used in clinical trials.
Speaker

Mark W. Tengowski, DVM, MS, PhD, Director, Medical & Scientific Affairs, Bioclinica
Dr. Tengowski brings a large breadth of applied medical knowledge, critical thinking ability and drug development experience from compound nomination through post-marketing investigations to Bioclinica. In the industry since 1999, his imaging experience spans ultrastructural- and light-level microscopic evaluations to non-invasive imaging methods, applying imaging endpoints to nominate and advance compounds, manage safety findings and achieve regulatory approval.
Mark served as a medical advisor to the Business Development team, supporting new requests for clinical trials and consultation while being a scientific expert for projects with an inflammation or musculoskeletal focus (e.g., osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, NAFLD/NASH and cholangitis, Crohn’s disease, ulcerative colitis, deep vein thrombosis, women’s health [breast volume/density, vaginal dryness, uterine fibroids, and endometrial thickness], medical devices, and orphan diseases [hyperoxaluria, acid sphingomyelinase deficiency, Pompe disease, Gaucher disease, Niemann-Pick disease]).
With a veterinary background in inflammation and pharmacology, Mark approaches protocols from a mechanistic point of view – devising an imaging strategy that will achieve the study’s objective and end points. In the external environment, Mark has been an invited speaker to the FDA and been the author of draft guidance issuances, given posters and podium presentations at national and international meetings, participated in several disease workshops, and published numerous abstracts, articles and book chapters. Dr. Tengowski is a graduate of the University of Wisconsin-Madison and is licensed to practice veterinary medicine in WI and NY states, with USDA-accreditation, and a member of the American College of Rheumatology.
Who Should Attend?
- Gastroenterologists
- Gut inflammation researchers
- Bowel medical affairs investigators
- Imaging technologists involved in gut image acquisition
What You Will Learn
In this webinar, participants will learn about:
- The inflammatory bowel distribution in Crohn’s Disease, ulcerative colitis and perianal fistula
- Advantages of magnetic resonance enterography in clinical assessments as well as clinical trials
- The common subject preparation, positioning, artifacts and general image acquisition pitfalls which can arise
- Examples of common MREn scoring features in CD and perianal fistula
Xtalks Partner
Bioclinica
Bioclinica is a global, life-science provider that utilizes science and technology to reduce risk of clinical trials – helping companies to develop new, life-improving therapies more efficiently and safely. The company’s offerings include medical imaging and cardiac safety services; clinical adjudication; randomization and trial supply management and optimization; electronic and eSource data capture; site and patient payments and budget forecasting; pharmacovigilance, and trial management.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account