The FDA has granted approval to Abeona Therapeutics’ Zevaskyn (prademagene zamikeracel, also known as pz-cel), marking it as the first and only cell-based gene therapy approved for the rare skin disease recessive dystrophic epidermolysis bullosa (RDEB).
The therapy is described as an autologous cell sheet-based gene therapy that acts as a skin graft.
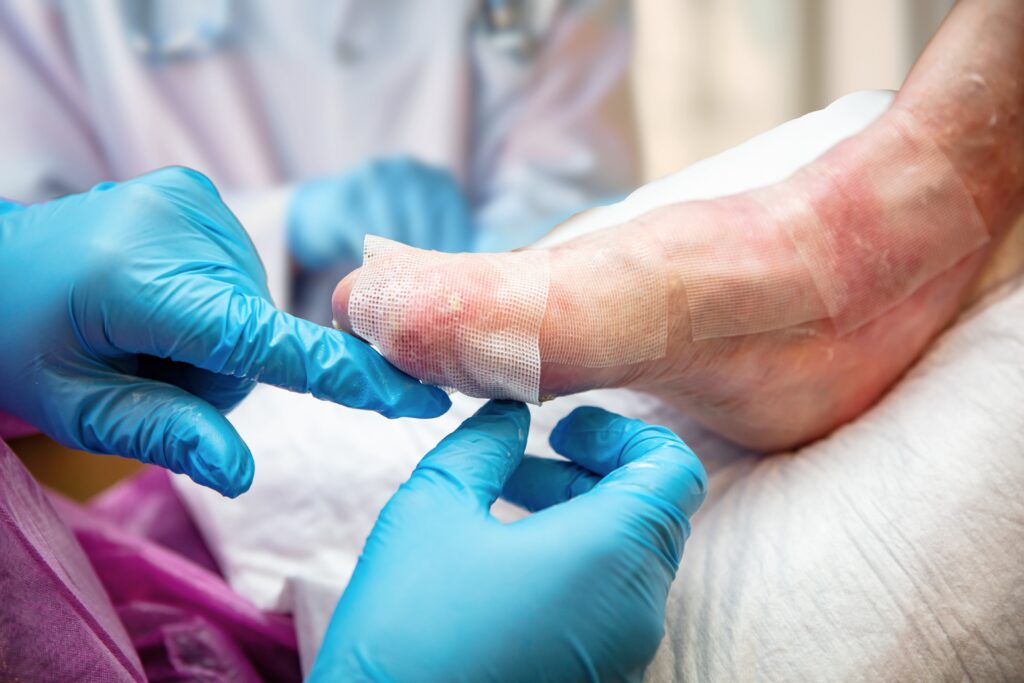
RDEB is a rare, debilitating skin disease characterized by extremely fragile skin that blisters and tears from minor friction or trauma, often referred to as “butterfly skin.”
It can lead to the formation of large, chronic wounds, which can cover over 30% — and in some cases up to 80% — of a patient’s body, resulting in intense pain and other complications, according to Abeona.
Patients also suffer from scarring and increased risk of aggressive skin cancers. Until now, treatment options were limited to palliative wound care, pain management and infection prevention, with no approved treatments targeted the underlying genetic cause.
In the US, RDEB affects approximately 2,000 to 2,500 individuals, many of whom face chronic pain, infections, disfigurement and shortened life expectancy.
XTALKS WEBINAR: Accelerating Patient-Centric Clinical Trials Through Science-Based Planning, Delivery and Support
Live and On-Demand: Monday, June 16, 2025, at 11am EDT (4pm BST/UK)
Register for this free webinar to learn strategic considerations for accelerating all studies, including complex and novel therapy trials. Attendees will gain insight into how to effectively integrate the patient voice throughout the clinical trial lifecycle.![]()
RDEB is one of the most severe subtypes of epidermolysis bullosa (EB), caused by mutations in the COL7A1 gene, leading to defective anchoring fibrils in the skin.
Zevaskyn is a disease-modifying treatment that delivers a functional COL7A1 gene into the patient’s skin cells (keratinocytes), aiming to restore the production of type VII collagen, a critical protein for anchoring the skin’s layers.
The ex vivo gene therapy involves harvesting a patient’s own skin cells, genetically modifying them in the lab to correct the COL7A1 mutation and then growing skin grafts that are transplanted back onto the patient’s wounds. The grafts not only reduce blistering and skin fragility but also promote durable skin regeneration.
“Today’s approval of Zevaskyn represents a pivotal moment in the treatment of RDEB, answering the call of people living with the clinical, economic and human impact of this devastating disease,” said Vish Seshadri, PhD, MBA, CEO of Abeona, in the company’s news release.
“We have heard from the RDEB community that there is a persistent unmet need to reliably address RDEB wounds, especially those that are chronic and prone to infection. Through a single surgical application, Zevaskyn can now offer people with RDEB the opportunity for wound healing and pain reduction in even the most severe wounds, as evidenced by the results from our pivotal Phase III study.”
Related: Unloxcyt Wins FDA Approval for Advanced Skin Cancer, Set to Compete with Keytruda
Zevaskyn’s approval was based on results from the pivotal Phase III VIITAL study. It met its two co-primary efficacy endpoints — a statistically significant healing of 50% or more from baseline in large chronic RDEB wounds, and pain reduction from baseline as assessed by the Wong-Baker FACES scale, assessed six months after treatment.
Specifically, among 43 large and chronic wounds treated with a single application of Zevaskyn, 81% of wounds showed 50% or more healing compared to 16% in 43 matched control wounds treated with standard of care.
Additionally, a single-center, open-label Phase I/IIa study involving 38 chronic wounds across seven patients showed that a single surgical application of Zevaskyn led to long-term improvement at treated sites over a median follow-up of 6.9 years (range of four to eight years).
Jean Tang, MD, PhD, professor of dermatology and lead principal investigator of the VIITAL study, explained, “In the completed Phase I/IIa study of Zevaskyn, we have observed wound healing and pain reduction that have lasted for years after a single application. Today we can celebrate the availability of an exciting new therapeutic option made possible by the incredible courage of patients and families who participated in these clinical studies.”
Abeona said that in both clinical studies, Zevaskyn was well-tolerated, with no treatment-related serious adverse events reported to date.
Abeona received a rare pediatric disease priority review voucher (PRV) linked to the approval, which it plans to monetize.
Abeona said it expects Zevaskyn to become available in the third quarter of 2025 through qualified treatment centers across the US.
Abeona also has a dedicated patient support program called Abeona Assist that provides personalized assistance, including guidance on insurance benefits, financial support options and travel or logistical arrangements for eligible patients.
Other players in the skin disease gene therapy space include Krystal Biotech, which is advancing the rollout of its topical gene therapy for dystrophic epidermolysis bullosa (DEB). Its treatment, Vyjuvek, received FDA approval in May 2023 and has since generated over $341 million in revenue.
If you want to have your organization featured on Xtalks, please email Ayesha Rashid at: [email protected]









Join or login to leave a comment
JOIN LOGIN