Spain-based orthopedics specialist company Abanza has announced the FDA 510(k) clearance of its QuadLock fixation system, a next-generation device designed to improve outcomes in anterior cruciate ligament (ACL) reconstruction surgery.
ACL injuries remain among the most frequently treated knee injuries in sports medicine, with approximately 300,000 reconstruction procedures performed annually in the US.
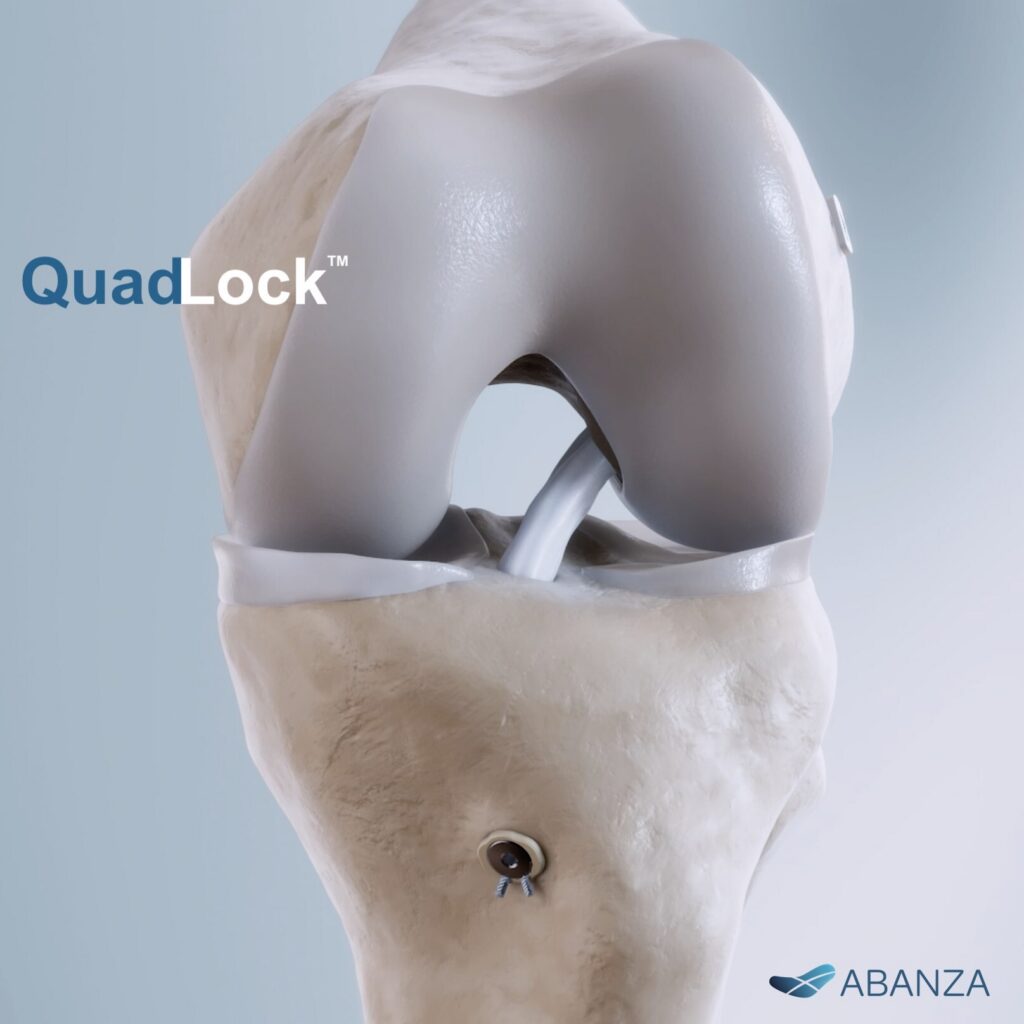
QuadLock is a knotless, bidirectional tension-adjustable fixation system engineered to securely affix soft-tissue grafts during ACL reconstruction. Its design enables surgeons to adjust graft tension precisely, a critical factor that influences early joint stability and long-term functional outcomes.
According to company-released data from rigorous biomechanical testing, QuadLock demonstrated less than 0.5 mm of cyclic displacement, representing a >500% improvement compared with traditional fixation techniques that typically show 3-6 mm of displacement under load. The system also delivered pull-out strength exceeding 1,000 N, highlighting its robust mechanical performance.
These performance metrics are important because excessive graft displacement and insufficient fixation strength can contribute to graft loosening and compromised knee stability, challenges that QuadLock aims to address.
Related: Regentis Biomaterials IPO Closes, Raising $10M to Support Pivotal Trial in Orthopedic Tissue Repair
The adjustable tension capacity of QuadLock allows it to accommodate a range of graft configurations commonly used in ACL reconstruction, including quadriceps tendon, quadrupled semitendinosus/gracilis and bone-patellar tendon-bone grafts. This versatility broadens its potential use across diverse patient anatomies and surgical preferences.
Following the approval, Abanza CEO Juan Abascal said: “QuadLock reflects our commitment to practical innovation, affording surgeons the adjustability, control and confidence needed during critical moments of fixation performance.”
“As our patient demographics evolve, traditional methods face increasing scrutiny, underlining the importance of our advancements.”
QuadLock joins a growing portfolio of Abanza fixation technologies designed to elevate surgical technique and improve patient outcomes. The company is actively developing complementary products, including LoopCap and WasherCap In Line systems, targeting a variety of procedures such as biceps tendon repair, acromioclavicular joint reconstruction and complex foot and ankle surgeries.












Join or login to leave a comment
JOIN LOGIN