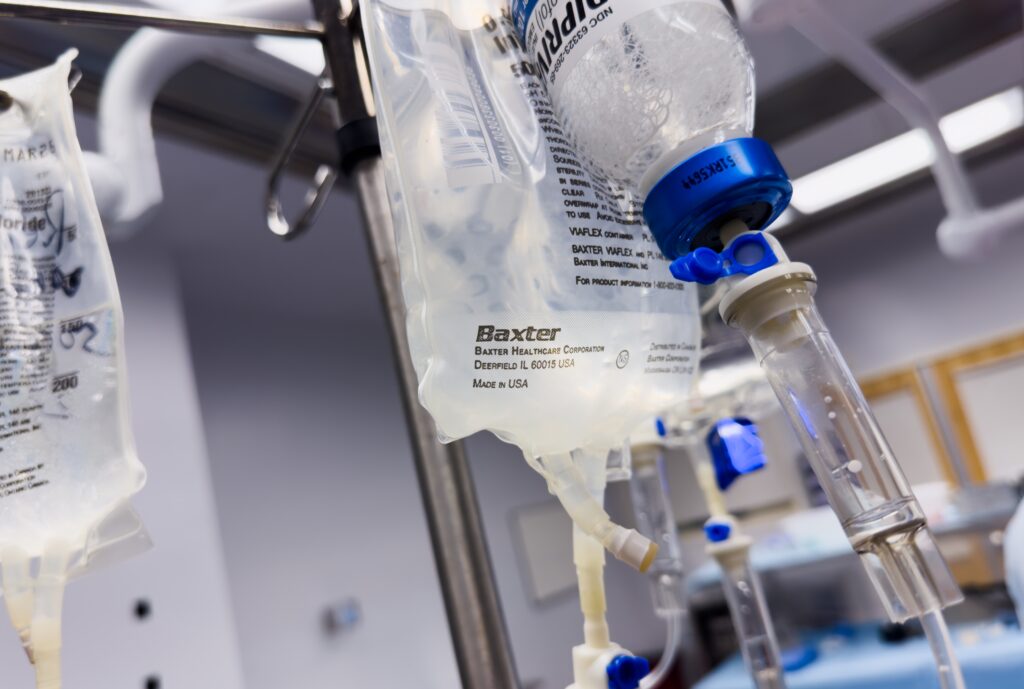
Leading medical devices and medical supplies company Baxter International announced it will restart a second production line for intravenous (IV) fluids at its North Cove, North Carolina facility impacted by Hurricane Helene.
The facility, a major supplier of IV fluids and Baxter’s largest manufacturing plant, was severely affected by the recent hurricane, leading to significant disruptions in production. The manufacturing site was significantly impacted by unprecedented rain and storm surges from Helene that led to water flooding the facility.
In the update issued on November 7, Baxter said it expects to restart the second line in the coming week. Last week, it restarted another line, and together, the two lines account for approximately 50 percent of the site’s total production. The company also said the lines produce about 85 percent of the site’s one-liter IV solutions, which is the most commonly used size by hospitals and clinics.
In a communication following its previous weekly update, Baxter said “barring any unanticipated developments, it expects to reach 100 percent allocation across several IV product codes by the end of the year.”
According to the American Hospital Association, the plant produces around 60 percent of the IV fluid used by US hospitals.
Baxter’s North Cove facility experienced flood damage from Hurricane Helene in late September, leading to a production shutdown. As a result, the Centers for Disease Control and Prevention (CDC) advised healthcare providers to prepare for potential supply disruptions that could impact patient care.
Related: Baxter’s Latest Update on North Carolina Facility in Hurricane Helene Aftermath
Bridges at the site were also damaged by Helene, leading to the installation of temporary bridges. Baxter said a second one had been installed at the site and is in operation, which will allow for additional truck and equipment traffic to enter and leave the site.
The company said its first temporary bridge had already moved over 1,000 truckloads of finished products off-site to customers.
In the update on Thursday, the company announced the next manufacturing lines to restart at the site will be for peritoneal dialysis (PD) solutions and irrigation, with production anticipated to be up and running again by early December.
During a conference call on Friday, CFO Joel Grade informed investors that Baxter anticipates a roughly $200 million impact on fourth-quarter sales due to disruptions from Hurricane Helene.
This includes approximately $40 million to $50 million in kidney care sales and $150 million to $160 million in sales of medical products and therapies, he outlined.
Baxter said the first batches are going to be manufactured along with “ongoing quality activities” and to ensure the quality and safety of the products will only be released in accordance with applicable regulatory requirements.
In terms of shipping timelines, the company said the earliest any new product from the North Cove site could start to ship is late November.
Baxter said this is ahead of its expectations, owing to the “diligence and resiliency of the North Cove and broader Baxter teams to accelerate recovery.”
Baxter said it was able to be ahead of schedule also because of coordination with the US Food and Drug Administration (FDA) and the Administration for Strategic Preparedness and Response (ASPR) within the Department of Health and Human Services (HHS).
To help boost inventory, Baxter has activated nine plants across its global manufacturing network.
According to the HHS, in mid-October, the FDA stepped in and performed scientific and regulatory evaluations to enable the temporary import of 23 different IV and peritoneal fluids from five Baxter facilities across the world to maintain domestic supply of IV solutions until the facility resumes full production. These facilities are currently ramping up production to increase the supply available to the US.
The HHS reported that the FDA had also “committed to expedite any and all necessary regulatory actions needed to help bring North Cove back on-line as quickly as possible.”
This includes accelerating reviews for enforcement discretion in additional situations, including evaluating requests from other manufacturers of IV and PD fluids.
Baxter also obtained FDA approval to extend the use dates of over 50 IV and irrigation codes, allowing up to an additional 12 months of expiry. These products now have a 24-month shelf life from the date of manufacture. This extension applies only to products manufactured before the end of September 2024, and details have been directly communicated to customers.
Baxter also transported over 450 truckloads of products produced before the hurricane out of the facility and into the supply chain.
In its latest update, Baxter outlined details on its planned, phased increases in allocations in late November, mid-December and end of year.
The company also shared that all 2,500+ of its North Cove employees were accounted for following Helene. Employees had returned to work across the facility, and remediation contractors also “remain engaged on a temporary basis to support site recovery.”
If you want your company to be featured on Xtalks.com, please email [email protected].










Join or login to leave a comment
JOIN LOGIN