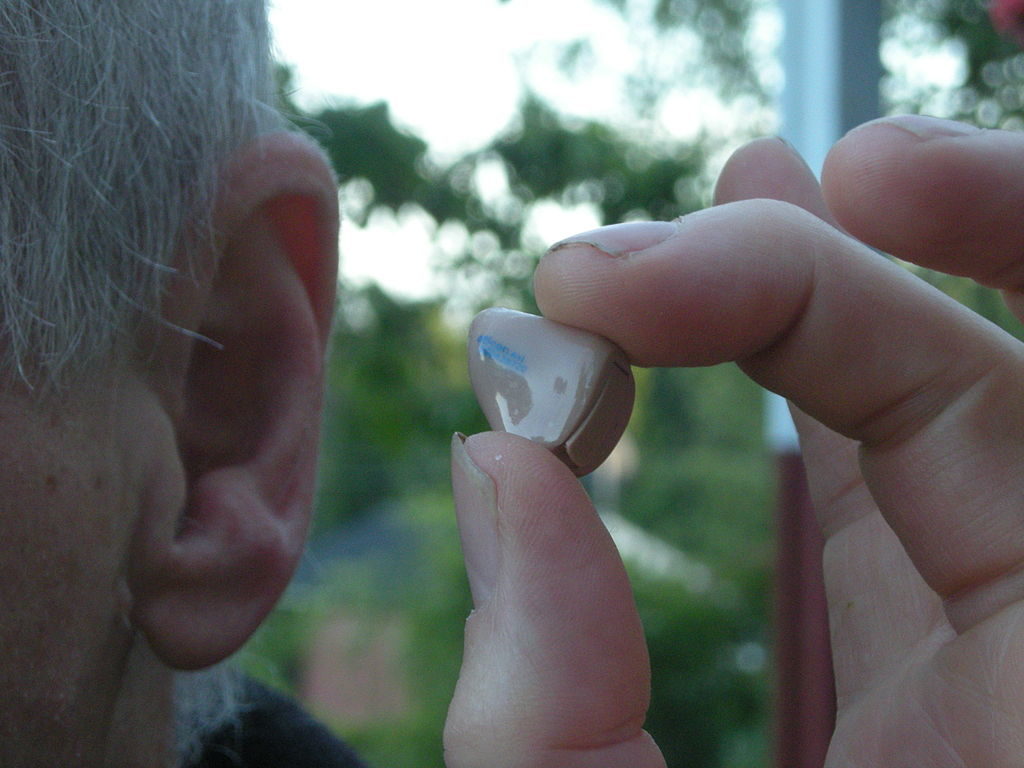
Bose is a household name when it comes to headphones and sound systems, however the American company just took a major step into the medical device market. The Bose Hearing Aid was just approved by the FDA as the first device which can be fitted, programmed and controlled entirely by the patient outside of the audiologist’s office.
“Hearing loss is a significant public health issue, especially as individuals age,” said Dr. Malvina Eydelman, director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA’s Center for Devices and Radiological Health. “Today’s marketing authorization provides certain patients with access to a new hearing aid that provides them with direct control over the fit and functionality of the device. The FDA is committed to ensuring that individuals with hearing loss have options for taking an active role in their healthcare.”
Adult patients with mild to moderate hearing loss will soon be able to take full control over regaining that sense thanks to the Bose Hearing Aid. It’s estimated that 37.5 million individuals in the US have at least minor hearing impairment, with some patients reporting a slight change in their ability to clearly hear, and others perceiving themselves to be almost completely deaf.
Medical conditions such as mumps, German measles and Usher’s syndrome can all lead to hearing loss, however aging and prolonged and repeated exposure to loud noises can also increase a person’s risk of developing a hearing impairment. In some cases, hearing loss is temporary, however individuals with permanent impairment can benefit greatly from a hearing aid.
As an air conduction type hearing aid, the Bose Hearing Aid uses external microphones to capture sound waves and amplify them through an earphone which is placed inside the canal of the ear. A mobile app allows users to adjust the hearing aid, making it the only device of its kind which does not rely on a healthcare professional to properly place and tune the device.
The approval brings the nearly $7 billion US hearing aid market closer to the availability of over-the-counter (OTC) devices which could be freely purchased by consumers from a variety of retailers. Right now, some states require hearing aids to be sold through a licensed dispenser, however the FDA is working on new regulations which could permit the sale of OTC devices in the future.








Join or login to leave a comment
JOIN LOGIN