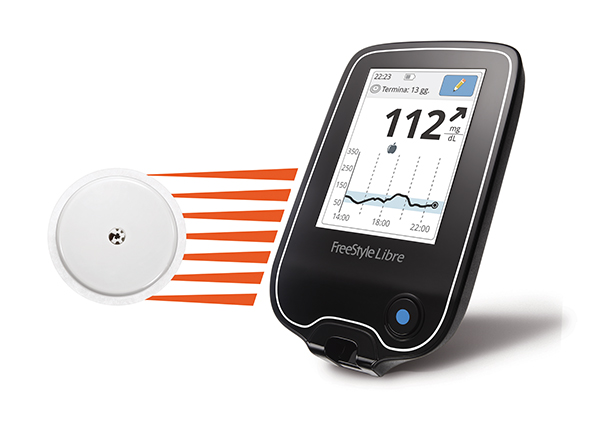
American patients with diabetes may never again have to use a finger stick to monitor their blood glucose levels thanks to Abbott’s new FreeStyle Libre Flash Glucose Monitoring System. The medical device was approved by the US Food and Drug Administration (FDA) this week, and is the first personal glucose monitor that does not require calibration using finger sticks.
“Today, we are celebrating a breakthrough moment for people with diabetes in the US—an end to the worry and hassles associated with routine finger sticks which have been the standard of glucose testing for more than 40 years,” said Jared Watkin, senior vice president, Diabetes Care, Abbott. “At Abbott, we believe that FreeStyle Libre will transform diabetes management and we’re proud to be at the forefront of innovation that empowers people to take control of their health to live their best lives.”
The device monitors glucose levels through a sensor attached to the back of a patient’s upper arm. According to Abbott, the device can be worn continuously for up to 10 days and costs much less compared to other continuous glucose monitoring systems on the market.
“The FDA is always interested in new technologies that can help make the care of people living with chronic conditions, such as diabetes, easier and more manageable,” said Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health and deputy director of new product evaluation in the FDA’s Center for Devices and Radiological Health. “This system allows people with diabetes to avoid the additional step of fingerstick calibration, which can sometimes be painful, but still provides necessary information for treating their diabetes—with a wave of the mobile reader.”
Diabetic patients who currently monitor their blood glucose levels using finger sticks may have to repeat the test as many as 12 times per day. However, most patients fail to comply with recommended testing schedules due to the pain and inconvenience of finger stick devices.
“Diabetes management requires active participation by the patient. Regular monitoring of glucose levels is especially crucial among patients being treated with insulin,” said Dr. Maria Tulpan, Lenox Hill Hospital, New York, N.Y. “What we see with the FreeStyle Libre system is patients gaining a better understanding of the impact of food, exercise and specific medications on their glucose levels due to availability of the data, which is important in the day-to-day management of diabetes and for behavioral changes towards improved diabetes control.”
Abbott says that the FreeStyle Libre Flash Glucose Monitoring System will be available by prescription in the US by the end of 2017. The device is already in use by diabetic patients in 40 other countries around the world.











Join or login to leave a comment
JOIN LOGIN