During the recent Xtalks webinar, “Implementing a Sound Logistical Strategy to Mitigate Risk and Streamline Clinical Testing Across Global Clinical Trials – A Central Laboratory Perspective,” I outlined several key considerations for conducting clinical testing in challenging locations across the world and explained how 3P logistics principles (people, processes, and plans) play an increasingly important role in helping navigate the complicated logistics of managing specimens during global trials.
Attendees were also invited to submit their questions on mitigating risk across global clinical trials, and I was able to provide the following insights during the live Q&A:
How do you handle the export of blood samples from China involved in a clinical trial? Is it forbidden?
It’s actually a common misconception that you cannot export blood samples in clinical trials. More focus should be given to the authorization to export, rather than the physical shipment itself. Getting this authorization to do so requires permission from the state drug approval administration in China, and there are various applications that need to be filled out. While it is possible, the whole process can take 10-18 months from start to finish.
How do you manage shipment of blood sample in ambient shipment from India to the US?
We partner with best-in-class providers that ensure sample stability from start to finish. They give us the option of providing temperature-controlled packaging to increase the longevity of each shipment. That way if delays do occur, the samples will still arrive at the destination at the appropriate temperature.
Do you have any experience with dry ice alternatives for shipping temperature-sensitive samples?
At ACM Global, we do use alternatives to dry ice shippers, mostly relying on systems containing phase change material panels. Although these alternative systems can be costly, they are reusable, they are reliable, and their smaller sizes reduce freight costs while increasing payload capacity.
Are there cost effective solutions available to monitor the temperatures of shipments in transit?
A cost-effective option would be to use a standard datalogger that, upon arrival, provides a summary of the various temperatures the shipment reached during transit. A higher priced option would be a sensor-based datalogger which provides the added benefit of having access to real-time data of each shipment throughout the entire journey. These sensor-based dataloggers provide alerts as soon as shipments exceed indicated temperatures, allowing real time intervention so they can be topped up with dry ice. Through a GPS tracking system, they can also provide constant updates on their geographic location, and they also provide other solutions such as a light sensor feature, which provides an alert if a shipment is opened during transit.
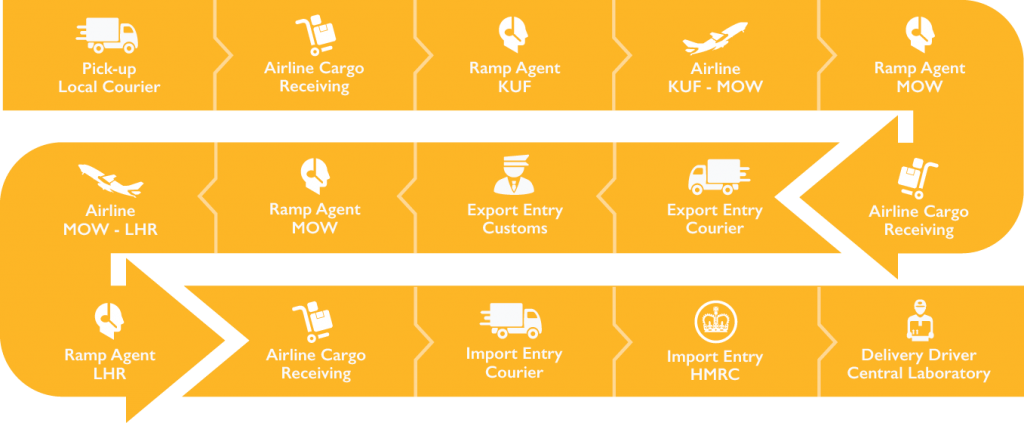
In addition to the Q&A, the session featured multiple choice poll questions. One of the more interesting results was the response to a question about how many different organizations/individuals attendees expected to be involved in a frozen shipment from Samara, Russia, to an EU central lab via specialist courier. While the majority of attendees thought the answer would be 10-15, the correct response is actually 15 or more. The various touch points are broken down in the chart below.
To learn more about this process flow strategy and to gain a greater understanding of how the 3P logistics principles minimize risk and improve operational efficiencies, you can access the full webinar on-demand in the Xtalks archive. You can download our new Smarter Testing e-book, which explores 6 principles for optimizing test selection, including engaging with laboratory experts on logistics early in the process and considering schedules, timing and logistics.
Feel free to share your opinions in the comment section below!






Join or login to leave a comment
JOIN LOGIN