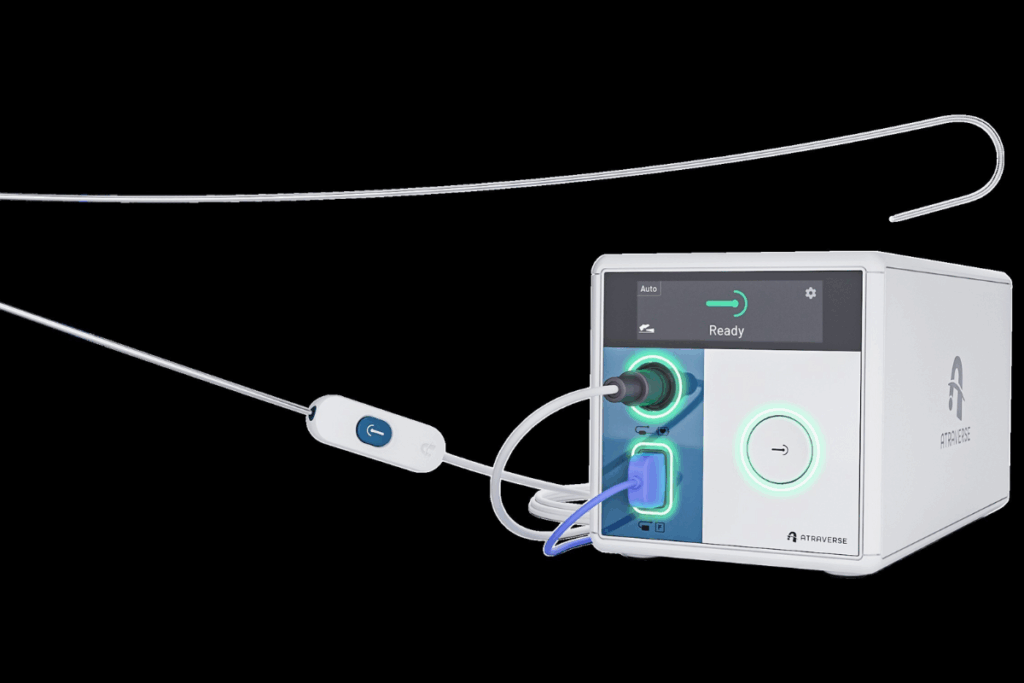
Atraverse Medical has received FDA 510(k) clearance for its fully integrated Hotwire transseptal access system. Hotwire is a radiofrequency (RF)-enabled platform designed to support controlled entry into the left atrium during electrophysiology and structural heart procedures.
Transseptal access involves crossing the thin wall between the right and left atria to deliver catheters used in procedures such as atrial fibrillation ablation. While widely performed, the step can be technically challenging, especially in patients with anatomical variability or prior interventions. It involves penetrating a thin internal wall, so tools that improve stability and visibility can help clinicians navigate the crossing more consistently.
Existing RF-based access platforms, such as Boston Scientific’s VersaCross exchangeless RF wire system and Medtronic’s FlexCath Cross integrated needle-and-dilator system, are already used in routine left-atrial access. They reflect the ongoing shift toward simplified, single-wire workflows.
Newer systems such as Hotwire build on this shift by integrating energy delivery, visualization features and a zero-exchange workflow into a single platform.
The Hotwire system combines an RF generator and RF guidewire to create a zero-exchange, sheath-agnostic workflow, meaning clinicians do not need to remove and replace devices once the septum is crossed. Once the wall is crossed, the wire remains in place and serves as the pathway for bringing other catheters into the left atrium.
The RF generator, introduced in August 2025, incorporates impedance-guided shutoff, a feature that halts energy delivery at the moment tissue is crossed.
According to the company, this approach is intended to reduce unnecessary RF exposure in the left atrium and allow operators to activate energy within the sterile field. Impedance-based control provides feedback on tissue contact, offering another layer of procedural guidance. The system uses this feedback to recognize when tissue has been crossed and to stop energy delivery automatically.
The RF guidewire, cleared by the FDA in May 2024, has been used in nearly 2,000 procedures. It includes a proprietary tip architecture reported to improve echocardiographic visualization by 25% and a reinforced core designed to offer twice the rail stiffness of leading competitors. Rail support refers to the guidewire’s ability to serve as a stable track for advancing sheaths and catheters, which can be important when introducing large-bore equipment.
This creates a stable route for advancing larger devices into position. The guidewire has demonstrated compatibility across 10 different introducer sheaths in early commercial use.
Clinical use so far has come from a limited market release rather than a single pivotal trial. Early operator feedback cited by the company points to the system’s potential to support more consistent transseptal crossing, particularly through enhanced visualization, wire stability and controlled energy delivery.
Safety information submitted for clearance reflects successful procedural performance with the generator’s automatic shutoff serving as an added safeguard. The known risks of transseptal access — such as perforation, pericardial effusion or arrhythmia — remain the same, and the system is intended to be used with standard imaging and clinical oversight.
XTALKS WEBINAR: Process AI in Medical Device: What Works and What’s Next
Live and On-Demand: Thursday, December 11, 2025, at 11am EST (5pm CET/EU-Central)
Register for this free webinar to learn how Process AI enables scalable, compliant automation for medical device manufacturers.
The clearance arrives several months after Atraverse Medical closed a $29.4 million follow-on financing in June 2025. Additional clinical experience and operator feedback have been expected to guide next steps as manufacturing capacity increases.
Use of transseptal access tools has been increasing as more left-atrial procedures, such as atrial fibrillation ablation and left atrial appendage interventions, become part of routine structural and electrophysiology care.
While Atraverse Medical’s device is now cleared, Protaryx Medical has submitted a 510(k) premarket notification for its next-gen transseptal puncture device after a first-in-human study in Paraguay, and is advancing toward potential US clearance.
Last year, the FDA cleared CIRCA Scientific’s CrossWise RF transseptal access system. It is now commercially available nationwide following a limited market release and uses a cannula-guided, zero-exchange design with expanded sheath compatibility for a broad range of left-atrial procedures.
If you want your company to be featured on Xtalks.com, please email [email protected].












Join or login to leave a comment
JOIN LOGIN