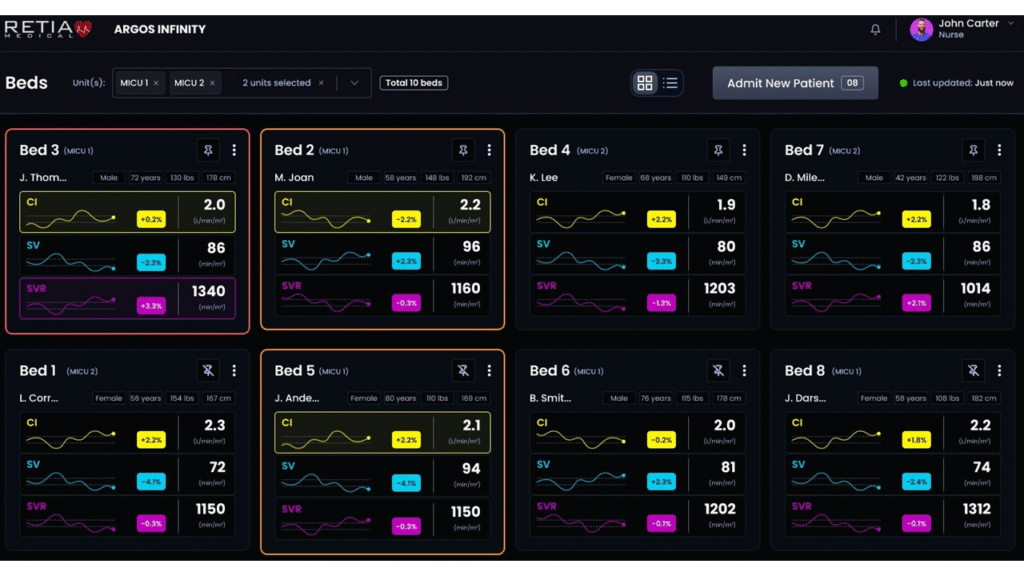
Retia Medical’s Argos Infinity has received FDA 510(k) clearance as a cardiovascular intelligence software platform. The platform is intended for use in high-risk surgical and critical care environments.
Argos Infinity is designed to support earlier identification of hemodynamic instability, which is when the heart struggles to supply enough blood and oxygen to the body. It is intended for use in adult patients in operating rooms, ICUs and tele-ICU settings.
Hospitals collect large volumes of patient data in surgical and critical care environments, including signals such as heart rate and blood pressure waveforms captured by bedside monitors. Clinicians often rely on individual numbers or short-term trends to assess whether blood pressure or heart rate falls within a normal range.
Argos Infinity analyzes that same monitoring data in a more detailed way. The software examines patterns across many heartbeats, and does not just focus on a single heartbeat. This helps provide insight into how effectively the heart is pumping blood and the overall stability of circulation.
In high-risk patients, cardiovascular deterioration can begin before routine measurements, such as blood pressure, show clear changes. According to Retia Medical, in cardiac surgery patients, nearly 70% of low cardiac index time occurs while blood pressure remains within the normal range.
When these early changes go unrecognized, patients may be more likely to require intensive care or experience organ injury, including acute kidney injury (AKI). AKI is one of several complications that can be associated with prolonged cardiovascular instability in hospitalized patients.
AKI is included as a hospital harm measure under the Centers for Medicare & Medicaid Services (CMS) programs. Hospital harm measures are used to assess hospital performance related to potentially preventable complications. The inclusion of AKI is expected to affect reimbursement in 2027.
Argos Infinity is built on Retia’s Multi-Beat Analysis algorithm, which evaluates cardiovascular performance across multiple cardiac cycles rather than relying on single-beat measurements or isolated vital sign readings. The approach is designed to function reliably in complex clinical situations, including arrhythmias, low cardiac output states and unstable blood pressure.
The algorithm underlying Argos Infinity has been evaluated in 14 peer-reviewed clinical publications. Before receiving FDA clearance, the software had been used under institutional review board approval across more than 400 beds in multi-hospital tele-ICU settings, operating alongside existing digital monitoring platforms.
Dr. Chiedozie Udeh, MD, Professor of Anesthesiology and Medical Director of Cleveland Clinic eHospital, noted that the software analyzes existing monitoring data hospitals already collect. This expands visibility into cardiovascular status without the need for new bedside equipment.
Over the past few years, patient monitoring systems have been incorporating software-based analysis of continuous physiologic data. In some cases, medtech companies have added AI and cloud-based analytics to existing monitoring platforms without requiring new bedside hardware.
In 2024, Medtronic added AI algorithms to its Reveal LINQ insertable cardiac monitor to reduce false alerts from long-term ECG data. The same year, BioIntelliSense’s wearable monitoring systems, designed to support continuous vital sign tracking across inpatient units and care-at-home programs, were cleared by the FDA. In perioperative and critical care environments, GE HealthCare expanded its monitoring portfolio in 2025 with software-based cardiac output and hemodynamic insights. These were aimed at making advanced cardiovascular monitoring available to a broader range of patients without invasive catheters or additional hardware.
Retia Medical said the FDA clearance allows it to expand the use of Argos Infinity beyond research settings. The platform complements the company’s Argos Cardiac Output Monitor, which uses the same algorithm at the bedside. In the US, the Argos monitor is distributed by Medtronic.
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN