Known for its AI-driven diagnostic tools, IMVARIA, a Berkeley-based health tech company, recently earned the FDA’s 510(k) clearance of ScreenDx, a first-of-its-kind algorithm designed to identify potential interstitial lung disease (ILD) cases. This follows their earlier success with Fibresolve, a tool for idiopathic pulmonary fibrosis (IPF) that became the first FDA Breakthrough-designated AI diagnostic for lung fibrosis.
AI-driven diagnostics have made waves in recent years, and 2024 was no exception. Thirona’s LungQ 3.0 received FDA clearance for its advanced thoracic CT image analysis, offering clinicians insights into lung structure and aiding in disease assessment. Similarly, Brainomix introduced e-Lung, an AI-powered tool that evaluates ILD by generating a weighted reticulovascular score, which quantifies scarring or inflammation in lung tissue to track disease severity and progression.
IMVARIA’s ScreenDx builds on this momentum, offering a distinct focus on early screening and referral pathways for ILD.
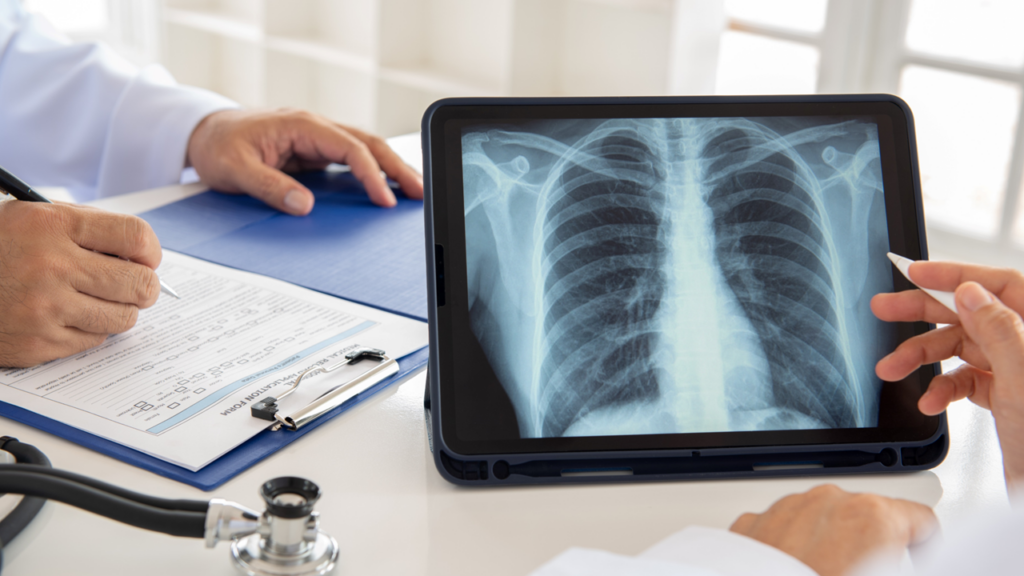
ILD — a group of disorders that cause progressive lung scarring, stiffness and reduced oxygen levels — often evades detection due to its complex symptoms and varied causes. The disease can lead to severe complications, including the need for lung transplants if not caught early.
Leveraging AI, ScreenDx analyzes computed tomography (CT) scans to detect patterns that may indicate interstitial lung findings, helping clinicians identify patients who require further evaluation.
According to Dr. Michael Muelly, IMVARIA’s co-founder and CTO, ScreenDx is designed to assist clinicians in diverse settings such as emergency rooms, lung cancer screenings or specialized clinics.
A supplementary tool, ScreenDx is built to integrate seamlessly into existing workflows. It enhances referral pathways by flagging potential cases earlier, ensuring patients are directed to the right specialists. Unlike traditional diagnostic tools, it is not intended to replace standard practices but to complement them, enabling faster and more informed clinical decision-making.
XTALKS WEBINAR: Transforming Lung Health and Disease using AI-based Registries
Live and On-Demand: Thursday, February 20, 2025, at 1pm EST (10am PST)
Register for this free webinar to gain insights into the power of AI-driven data analysis to identify at-risk patients, optimize clinical trials and accelerate innovation in pulmonary disease care.
Aside from AI-based diagnostics, researchers have been exploring genetic testing to identify hereditary risks, liquid biopsies for analyzing circulating DNA and advanced imaging techniques like endobronchial optical coherence tomography for visualizing lung tissue at a high resolution.
Non-invasive tools like electronic nose (e-nose) technology are also being studied to analyze breath compounds and differentiate between ILD subtypes.
IMVARIA’s solution aims to upgrade the diagnostic toolkit. By addressing diagnostic delays, ScreenDx could make a significant impact on patient care, ensuring timely interventions and better management of this debilitating condition.
Affecting around 650,000 people in the US and responsible for tens of thousands of deaths annually, ILD begets the need for early detection. And IMVARIA is rising to the diagnostic challenge, addressing delays in diagnosis and ensuring patients are referred to specialists more efficiently.







Join or login to leave a comment
JOIN LOGIN