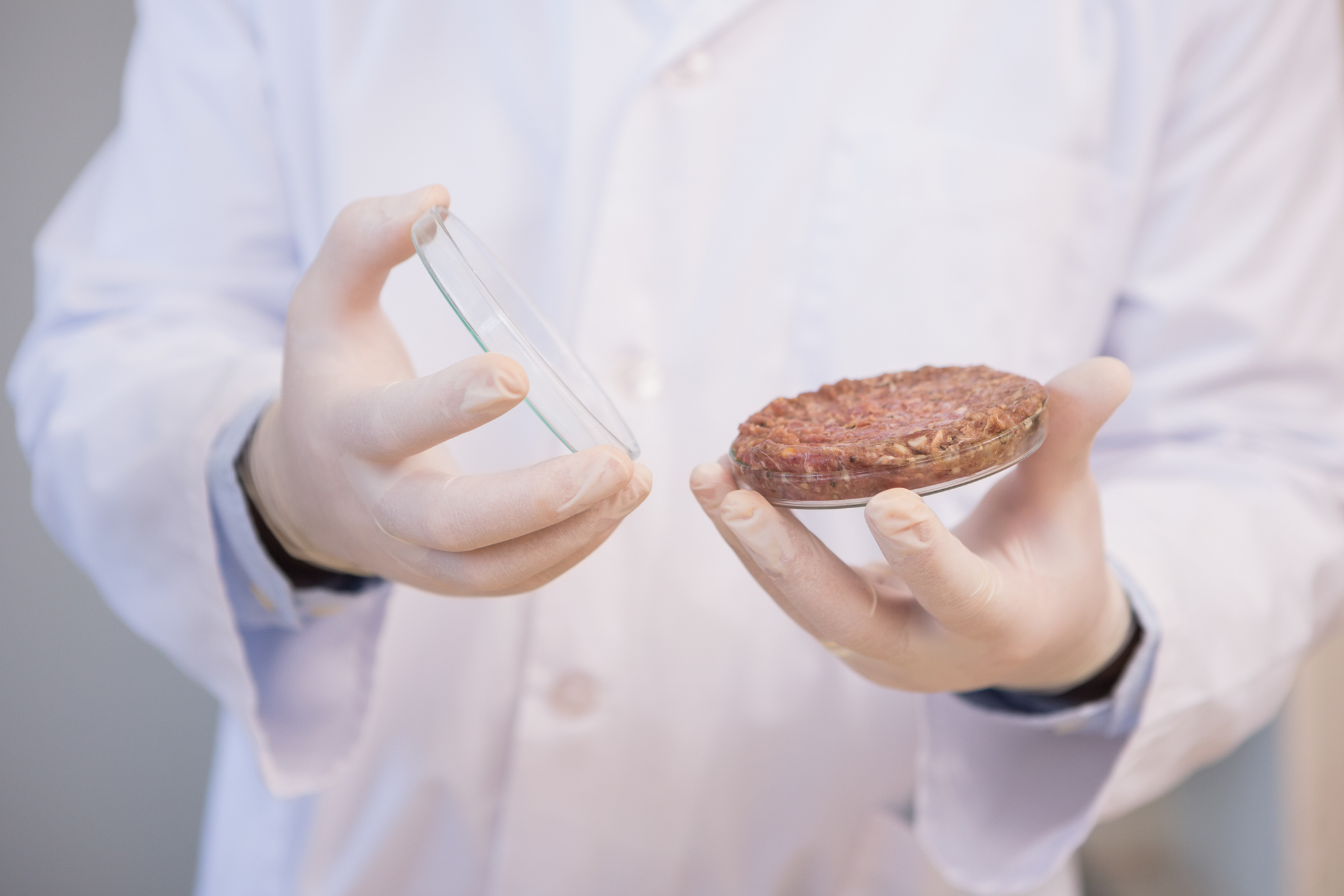
Lab-grown meat could change the way we produce and consume food. Now, researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have grown rabbit and cow muscle cells on edible gelatin scaffolds to replicate the texture and consistency of meat.
On Monday, the team of researchers revealed their findings in npj Science of Food showing success and progress towards changing the way consumers see their meat. Senior author Dr. Kevin Kit Parker, a core faculty member of the Wyss Institute and the Tarr Family Professor of Bioengineering and Applied Physics at SEAS, told Xtalks that the inspiration for this research came from a Winston Churchill quote:
“We shall escape the absurdity of growing a whole chicken in order to eat a breast or a wing, by growing these parts separately under a suitable medium.”
Click the play button to listen in on the conversation
Dr. Kit Parker also serves as a consultant to the food industry and was invited to be a judge on a Food Network show. There, he was talking to some of the chefs and he “started thinking there’s no reason why we couldn’t take everything that we applied to regenerative medicine and apply it to make it meat.”
The biggest issue that they had was trying to figure out how to create the perfect texture for their lab-grown meat. They used a technique called immersion Rotary Jet-Spinning (iRJS) that is also used for wound dressing and heart valves. Their technique uses centrifugal force to spin long nanofibers, making the muscle scaffold for the meat. They needed to make a protein that had a solvent but would not break down in water. After some research they knew that gelatin is a metabolized form of collagen. Therefore, they used collagen, a protein that holds most skeleton muscle together, and made protein fibers out of gelatin.
According to Dr. Kit Parker, this process works by putting collagen in the iRJS machine with ethanol and letting it spin, so they could spin fibers out of gelatin and into the ethanol. The ethanol is then allowed to evaporate leaving only the gelatin fibers. They then cross link the fibers and add the cells (taken from rabbits or cows) that grow and divide and make a piece of lab-grown meat.
Dr. Kit Parker says one of the most important parts about meat is its texture and taste. Therefore, they focused on spinning the fibers out of collagen to make gelatin in order to create the best mimicked texture of real meat. After various tests, Dr. Kit Parker and his team brought in a bunch of meat and tested the chewability in comparison to their lab-grown meat and say they have now figured out the accuracy in texture and want to focus on taste next.
Dr. Kit Parker said, “part of the taste when you eat a piece of steak is because of the fat, and the different kind of fat that is marbled inside the meat, and we’re going to be able to control for that. So, the next step is, can we build fat into this meat? We have solved texture. Can we get taste? That’s what we’re intending to do.”
Another concern that the researchers at SEAS were facing was time. It takes a long time to make these scaffolds. It takes around 45 days to grow lab-grown chicken meat and most engineered meat takes even longer. Nevertheless, they are now making meat in around 40 days. The reason that this process takes a long time is because they must wait for the cells to multiply in order to make the meat.
The lab-grown meat produced in the lab does contain animal cells, but Dr. Kit Parker says that this technique could be replicated to make a lot of plant-based products with better texture and taste compared to what we are seeing today.
As for the future of lab-grown meat, Dr. Kit Parker has a specific goal in mind.
“I want to eat a filet mignon made in a lab and I don’t want it to increase my cholesterol,” concludes Dr. Kit Parker.









Join or login to leave a comment
JOIN LOGIN