Medical imaging tech company Polarean Imaging has recently announced that the US Food and Drug Administration (FDA) has approved their drug-device combination product Xenoview (xenon Xe 129 hyerpolarized). Xenoview is indicated for use with magnetic resonance imaging (MRI) to evaluate lung ventilation in adults and pediatric patients who are at least 12 years old.
According to Polarean, Xenoview is the first and only inhaled hyperpolarized MRI contrast agent to reach the market.
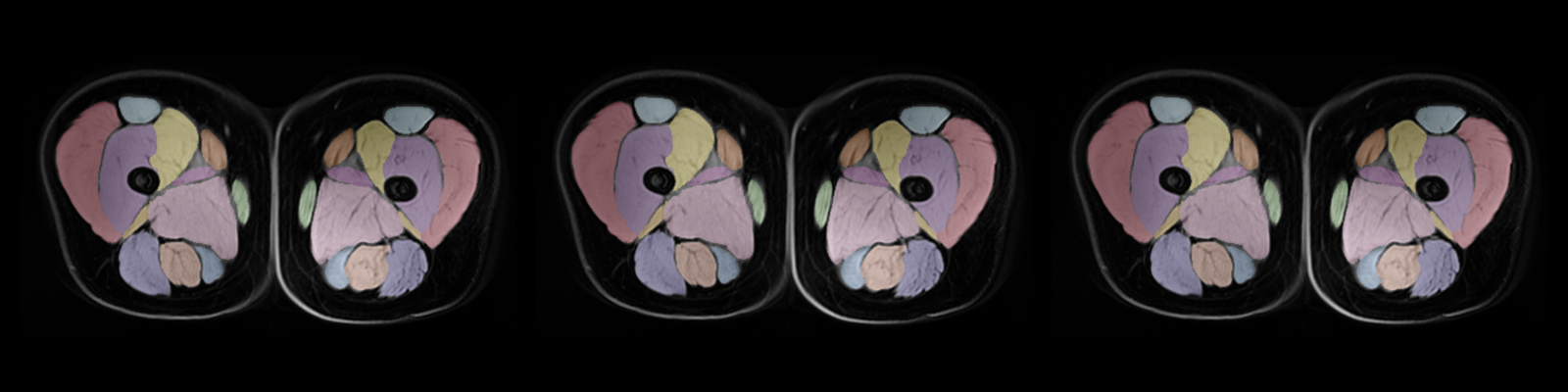
Hyperpolarized gas diffusion — using isotopes of xenon (129Xe) — greatly increases the contrast and sensitivity of pulmonary MRI and provides a reliable method to detect lung disease.
Xenoview is a 129Xe gas blend; it is packaged in a dose delivery bag and thus can be inhaled by a patient during a single breath hold MRI procedure (10 to 15 seconds long). Xenoview is produced by a device called the Xenon Hyperpolarizer, which uses a mixture of unpolarized xenon, nitrogen and helium to yield hyperpolarized xenon. After hyperpolarization, it is packaged into a dosage delivery bag.
The benefit of Xenoview over other lung ventilation assessment technologies like computed tomography (CT) scans is that Xenoview allows for novel visualization of lung ventilation without exposing patients to any ionizing radiation.
In the US, about 37 million people live with chronic lung disorders such as chronic obstructive pulmonary disease (COPD) and asthma. Xenoview provides a regional map of a patient’s lung ventilation and can help pulmonologists, respiratory specialists and surgeons manage their patient’s lung disease.
“FDA approval represents achievement of a major milestone for Polarean’s technology. This was only possible in close collaboration with multiple research clinicians and scientists globally, who we thank for their tireless and enthusiastic work,” said Richard Hullihen, Chief Executive Officer of Polarean in the company’s press release.
“Approval of Xenoview represents a major step forward in modern respiratory imaging and we are proud to have pioneered this exciting new technology for clinical use. The commercial team at Polarean is prepared to rapidly launch XENOVIEW for clinical application,” added Hullihen.
XTALKS WEBINAR: Patient Journey and Disease Progression — Exploring the Power of Images in Real-World Data
Live and On-Demand: Wednesday, January 25, 2023, at 1pm EST (12pm CST / 10am PST)
Register for this free webinar to hear use cases for imaging that include support for clinical trials, optimized dosing schedules and comparative effectiveness studies.
Two More Devices Approved by the FDA Alongside Xenoview
Polarean has also obtained the FDA’s approval on two major devices to support the launch of Xenoview into the clinical marketplace.
One of the devices is an image processing software called Xenoview VDP, which can quantify xenon intensity using a pulmonary hyperpolarized 129Xe MRI and an accompanying proton chest MRI. This software allows clinicians to visualize and evaluate a patient’s lung ventilation.
The other device is called the Polarean Xenoview 3.0T Chest Coil, which is a single channel radiofrequency coil that is tuned to the 129Xe frequency. The chest coil is worn by the patient and is used with the inhaled Xenoview contrast agent and compatible 3.0T MRI scanners to evaluate lung ventilation.
Xenoview Clinical Studies
Two prospective, multi-center, randomized, open-label, cross-over clinical trials compared Xenoview MRI to 133Xe scintigraphy in adult participants with pulmonary disorders.
Study 1 compared Xenoview and 133Xe imaging in 31 patients being evaluated for possible lung resection surgery and who have a medical history of respiratory disorders like COPD and pulmonary mass. The mean within-patient difference in the predicted postoperative percentage of remaining lung ventilation between Xenoview and 133Xe imaging was within a pre-specified equivalence interval.
Study 2 compared Xenoview and 133Xe imaging in 49 patients being assessed for possible lung transplant surgery with a medical history of respiratory disorders like COPD, interstitial lung disease, idiopathic pulmonary fibrosis, and others. The mean within-patient difference of overall lung ventilation contributed by the right lung between Xenoview and 133Xe imaging was within a pre-specified equivalence interval.
“My colleagues and I in the Xenon MRI research community are thrilled that this technology is now available to reach both adolescent and adult patients. With the availability of Xenoview in the clinical setting, we will be able to evaluate regional lung ventilation, delivered with a benign safety profile, which has been a major unmet need for the patients that look to us to better understand their lung disease,” said Dr. Jason Woods, Director of Research in Pulmonary Medicine at the Cincinnati Children’s Medical Center, in the company’s press release.











Join or login to leave a comment
JOIN LOGIN